THE ESSENCE OF THE INFORMATION 👑
👸 👑 🤴
Graphene oxide in the form of black fibers is known to be found on some medical masks.
These fibers are called "Morgellon worms", they react, like living, to breathing - changes in humidity and temperature.
There is a centurion of videos on YouTube, where this is recorded.
And also they are on sticks for smears, and on other objects, even on our skin.
According to numerous analyses conducted by the State University Laboratory (UAL - Universidad de Almeria), all Pfizer Anti-Covid injection studies contain significant amounts of graphene oxide.
(Breaking News: La Quinta Columna shares the official interim report of the analysis of Pfizer's vaccination vial).
Also, the influenza vaccine used in the fall of 2019 contained graphene oxide.
The same goes for prepared new flu vaccines and suspected intranasal Covid-19 vaccines, which contain very high amounts of graphene oxide.
Graphene oxide is a toxic substance that causes the formation of blood clots in the human body.
Graphene oxide causes blood coagulation.
Graphene oxide – by disturbing the oxidative balance with respect to glutathione stores (practically causing oxidative stress) – changes the functioning of the immune system.
Graphene oxide, introduced into the body, causes the collapse of the immune system, and then the so-called cytokine storm.
Graphene oxide, which accumulates in the lungs, causes bilateral pneumonia.
Graphene oxide is felt in the mouth as a metallic taste.
Inhalation of graphene oxide causes inflammation of the mucous membrane, and what is associated with this: loss of taste, partial or complete loss of smell.
Graphene oxide acquires strong magnetic properties in the human body.
And that's what explains the phenomenon of body magnetization that millions of people around the world are filming after they received doses of graphene oxide as a result of various ways of administering it, including through Covid "vaccines."
A particularly strong interaction is shown by the aerosol of graphene oxide.
Thus, graphene oxide causes some of the symptoms of SARS-COV-2.
5 G RADIATION AND GRAPHENE OXIDE
The installation of 5G antennas during the "pandemic" was not suspended for a moment.
We suspect that graphene oxide was introduced into the flu vaccine used during the 2019 campaign, as graphene oxide was previously used in vaccines as an adjuvant.
With the advent of subsequent technological experiments involving 5G technology around the world, the disease Covid-19 has spread, which is explained by the interaction of external magnetic fields with graphene oxide present in the bodies of sick people.
Note that it all began in Wuhan, that is, in the world's first starting city, where 5G technology was tested at the end of November 2019.
Both time and place coincide.
Both the story of the lizard and the bat soup were intended to divert public attention from the real danger.
So, covid's severe course is not a biological factor, but an additive (the icing on the cake) - the action of a toxic chemical.
Severe cases of Covid 19 are often a side effect of nanotechnology on the human body.
Source:
This is the official preliminary report from the University of Almeria, signed by Prof. Dr. Pablo Campra Madrid.
About Pfizer vaccines.
WHAT FOR?
The goal of a small group of state biological terrorists: the change of the world order and the establishment of war communism on the basis of false medical fascism through the introduction of the most severe control of the individual and the population, as well as the behavior of society. De facto the elimination of nation-states and the usual way of life, the abolition of private property and many other nishtias characteristic of war communism.
Graphene oxide nanoparticles contained in false vaccines cross the blood-brain barrier and reach the brain.
These nanoparticles, subjected to remote excitation using 5G, are able to modify neural synapses.
Thus, something very similar to the "tsunami of the brain" caused allegedly by the coronavirus is expected.
Scientists warned about this, we wrote about it for many months in a row.
(Scientists warn ‘synaptic tsunami’ in COVID-19 patients )
June 3, 2021 3 min read
https://www.entrepreneur.com/article/373645
This article was translated from our Spanish edition using AI technologies. Errors may exist due to this process.
More than a year after the COVID-19 pandemic began, we continue to learn about this disease and it is that recently many patients reported continuing to feel discomfort such as fatigue, shortness of breath and even anxiety after months of having suffered it.
***
What do people allow to enter through injection?
De facto, together with an injection in the hand of unknown injection formulations, people receive a poisonous ☠️ effect of the interaction of graphene oxide in the false vaccine 💉 and exposure to 5G technology - video ⬇️
https://t.me/Tribulelouis/8871
https://t.me/Tribulelouis/8882
https://t.me/Tribulelouis/8827
Graphite oxide (graphene oxide) is a compound of carbon, hydrogen and oxygen in various ratios, which is formed during the processing of graphite with strong oxidants . The most oxidized forms are solid yellow substances with a ratio of C:O in the range from 2.1 to 2.9.
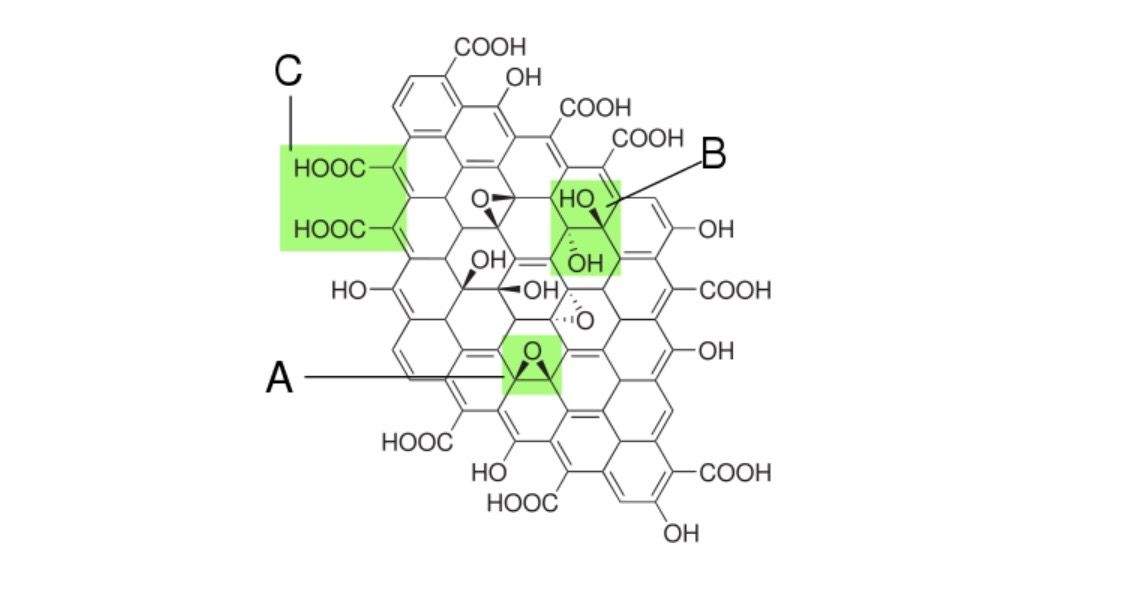
Structure proposed in 1998 with different functional groups (A: epoxy, B: hydrooxyl, C: carboxyl).
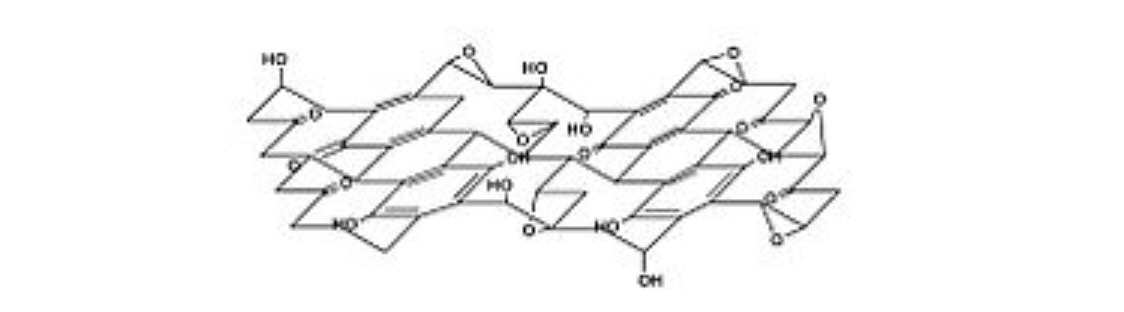
Decant formula

Nakajima-Matsuo formula
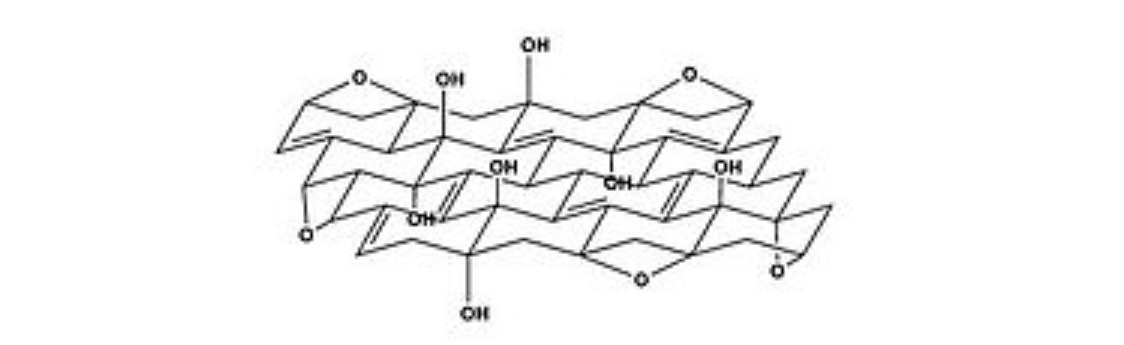
Ress formula

Scholz-Boem formula
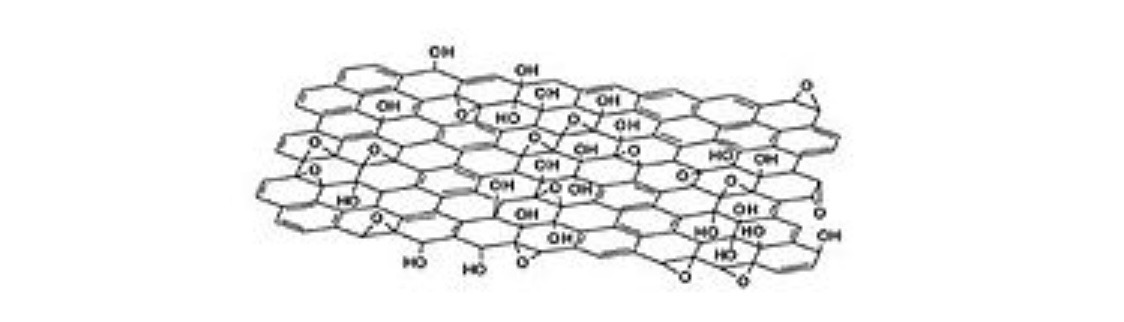
One sheet of crystal lattice of graphite oxide. Several oxygen-containing functional groups are shown. Absolute and relative numbers of functional groups depend on the specific method of synthesis (Lerf-Klinovsky structure)
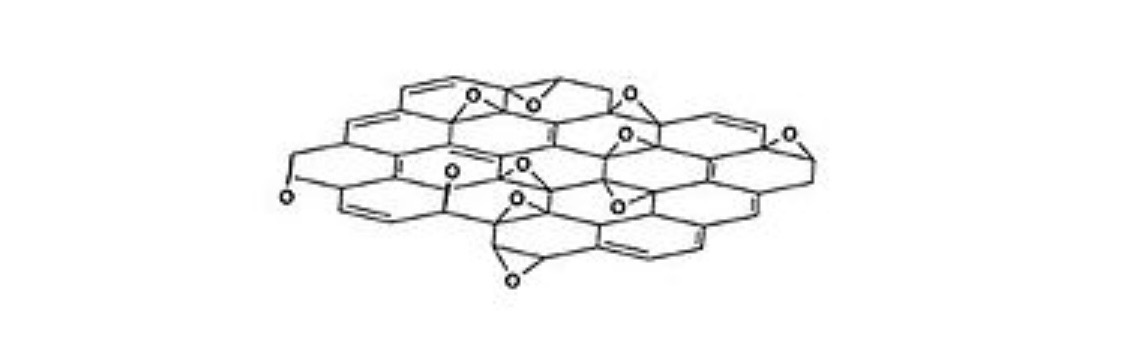
Hoffman's Structural Model
There are many models of the structure of graphite oxide. This is due to the fact that it is a berthollid and has a difficult amorphous structure, as well as the lack of analytical methods to characterize such materials.
The Hoffman structure consists of epoxy groups distributed over a plane and attributes the oxide to the formula C2O.
Ress proposed another option using hydroxyl groups. The basal plane evolved from sp2 to sp3 hybridized system. This formula was created as an analogue of that in the polymer carbon monofloride.
The Scholz-Boem model does not contain epoxy groups.
The structure of the Decanter is a modification of the previous two.
The Nakkajima-Matsuo structure is an analogue of the polymer dicarbon monofloride.
The latest Lerf-Klinovsky model is more focused on a non-sterichiometric, amorphous alternative.
The main part of graphite oxide is used to prepare a dispersed system with alkalis to produce monomolecular sheets, which are called graphene oxide (by analogy with graphene, which is a single-layer form of graphite).
Sheets of graphene oxide have been used to create a very strong material that resembles paper, and as an intermediate product for producing graphene (as of 2010, this is not possible, as the graphene produced by these reactions still has many chemical and structural defects).
History
Graphite oxide was first prepared by Oxford scientist Benjamin Brody in 1859 by treating graphite with a mixture of potassium chlorate and nitric acid.
In 1957, scientists William Hammers and Richard Offerman found a more reliable, faster and efficient process using a mixture of sulfuric acid H2SO4, sodium nitrate NaNO3 and potassium permanganate KMnO4. This method is still widespread and is still used for the synthesis of graphite oxide.
Recently, a mixture of H2SO4 and KMnO4 was used to longitudinally "cut" carbon nanotubes, resulting in microscopic flat tapes of graphene several atoms long, with "roofs" of oxygen atoms or hydroxyl groups.
Graphite oxide can also be prepared using the Tan-Lau method, which uses glucose. This method is safer, lighter and more environmentally friendly compared to traditional reactions using strong oxidants. Another important advantage of the Tan Lau method is the easy thickness control.
Structure
The structure and properties of graphite oxide depend on the specific synthesis method and the degree of oxidation. Usually, layers are preserved, as in graphite, but the distance between them increases by about two times (~0.7 nm) compared to graphite. Strictly speaking, "oxide" is a misnomer, but historically established name. In addition to epoxy groups, there are other experimentally established functional groups, for example, carbonyl, hydroxyl, phenolic. There is evidence of "bending" and cracking of graphene oxide sheets during the deposition of layers on the substrate. The detailed structure is still not clear due to severe frustration and irregular packaging of layers.
The thickness of the graphene layers of oxide is about 1.1 ± 0.2 nm. With the help of tunnel microscopy, local regions were found where oxygen atoms are located in a constant lattice of 0.27 nm × 0.41 nm, the edges of each layer are cut off by carboxyl and carbonyl groups. X-ray photoelectron spectroscopy shows the presence of carbon atoms in rings that do not contain oxygen (284.8 eV), C-O (286.2 eV) in C=O (287.8 eV) and in O-C=O (289.0 eV).
Graphite oxide is easily hydrated, resulting in an increase in the inter-flat distance (up to 1.2 nm in the saturated state). Additional amounts of water are also included in the layer due to the high pressure of the induced effects. The main product absorbs moisture from the surrounding air in proportion to the humidity. Complete removal of water is very difficult, since heating at 60-80 ° C leads to partial decomposition and degradation of the material. Like water, graphite oxide also easily includes other polar solvents, such as alcohols (as well as DMFA and N-methylpyrrolidone).
The separation of graphite oxide layers is proportional to the size of the alcohol molecule, additional monolayers are inserted into the structure at high pressure.

Let's all pour 96* , Baby )
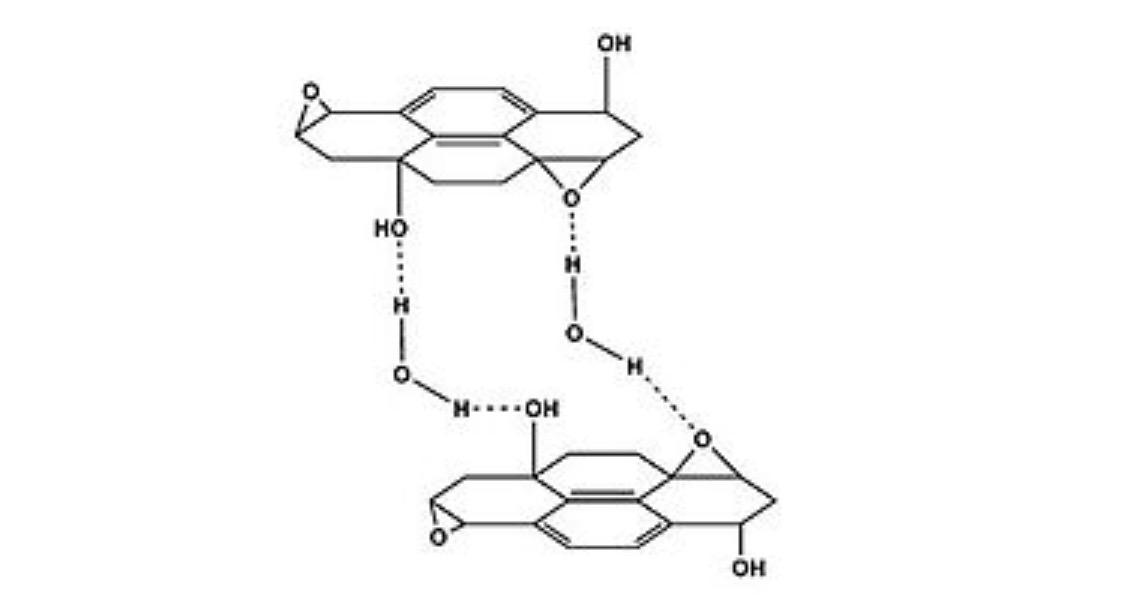
Proposed hydrogen bridges between graphite oxide and water
Graphite oxide decomposes under rapid heating at moderately high temperatures (~280-300 °C) to form finely dispersed amorphous carbon, slightly similar to activated carbon. Soot consists of the thinnest graphite scales with a thickness of 2-5 nm, the diameter of which can reach several hundredths of a millimeter, which depends on the nature of the original graphite. Since this releases oxygen bound in the form of CO and CO2 in graphite oxide, it is possible that voids of atomic size arise in the graphite lattice.
Due to the specific two-dimensional structure and the existence of different oxygen-containing functional groups, graphite oxide has many applications in a wide variety of areas.
Super Capacitors
Potassium hydroxide restructures graphite oxide, creating a three-dimensional porous structure. Each of its walls has an atomic thickness, and the surface area of the "activated" graphite oxide reaches 3100 m² / g. The material also has a high specific electrical conductivity. The diameter of most of the pores in the finished samples falls into the range of 0.6-5 nm. In experiments, a supercapacitor built using a new electrode material showed very good gravimetric capacitance and energy density, with the latter approaching the performance of lead-acid batteries. After 10,000 charge/discharge cycles, the "activated" graphite oxide continued to operate at 97% of its original capacity.
Heavy-duty paper
When dissolved in water, graphite oxide is exfoliated into layers of graphene oxide. The resulting solution is filtered through a special membrane, on which the layers are again bound, but already in a much stronger structure than graphite - graphene paper. Layers of ordinary graphite are very weakly connected and the breakage of bonds occurs easily. In graphene paper, on the contrary, the layers are intertwined, so the load can be distributed evenly throughout the structure, making it very strong. The way the layers intertwine allows them to shift slightly relative to each other, making the entire structure flexible. More importantly, it is possible to chemically control the properties of a given material by changing the amount of oxygen in the layers. For example, by reducing it, you can make paper from a dielectric a good conductor. It is also planned to introduce various polymers and metals into the structure of graphene paper, creating composites that surpass both pure graphene and dopant in their properties.
DNA studies
The large flat surface of graphene oxide allows multiple DNA probes labeled with different dyes to be examined simultaneously, providing detection of multiple target DNA in the same solution. Further progress in the search for sensors from graphene oxide and DNA may lead to the creation of inexpensive rapid DNA analysis systems 🧬. In medicine for the treatment of cancer of the brain, thyroid gland, etc.
The largest producer of graphene is located in China. This is Ningbo Morsh Technology, founded in 2012. Last year, it launched the world's largest 300-ton graphene line. The main consumer was a sister company Chongqing Morsh Technology, which uses graphene to produce 2 million pieces / g of transparent conductive films for mobile phones.
Features of graphene oxides
The term "graphene oxides" has not yet received an international definition. Graphene oxides are understood as graphene particles with oxygen-containing functional groups and/or molecules attached at the edges or inside the carbon grid. The nomenclature of these groups is extensive: hydroxyl, phenolic, carbonyl, carboxyl, aryl, ether, phosphorus-containing, etc.? p. A variety are graphene oxides modified with polymers such as polyethylene glycol, polyesters, polyvinyls, polyacryls, etc. Another group of graphene oxides are doped compounds. In particular, graphene oxides containing in their structure one or more atoms of boron, nitrogen, aluminum, phosphorus, silicon, sulfur or groups based on them, for example, melamine, phosphine, silane, polysiloxane, sulfides, etc. are known.
The most beautiful graphene oxides are obtained by incorporation of crown esters molecules [03]. At the end of 2014, they were received in the famous US nuclear development research center - Oak Ridge National Laboratory. The size and shape of the cavity formed by the crown ether molecule depend on its composition. Therefore, the new material can sorb ions of a strictly defined diameter. The strong electrostatic bonds of ether molecules incorporated into the graphene network offer enticing prospects in biotechnology for chemical separation, metal extraction, radionuclide purification, rare earth recycling, and data storage.
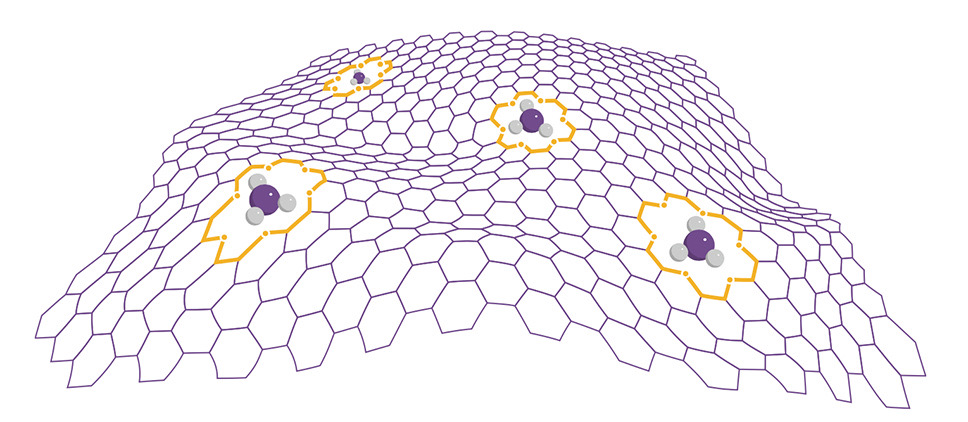
Structure of graphene with incorporated crown ether molecules
In general, graphene oxides in terms of sorption capacity are significantly superior to polymer-based ion exchange resins and other traditional sorbents. This is the essence of interest in graphene oxides to create super-sorbents of a new generation.
Sorption records of graphene oxides can be realized in several ways, for example, by absorption; adsorption; ion exchange; physical adsorption; cemosorption; with the establishment of covalent or non-covalent bonds; with the establishment of hydrogen bonds; Van der Waals interaction.
As a result of sorption, colloids can be formed, coagulation of the substance and the subsequent formation of precipitation can occur.
Specialists know five main varieties of graphene oxides in the form of particles:
films on inert substrates;
nanopowders with a size of flat particles (scales) of about 905 nm;
flakes with a particle size of 1-5 μm;
tapes (with a ratio of length to width of more than 10);
pompons with a size of spherical particles with a diameter of 3-6 μm.
The most unusual are pompons, that is, splices of graphene petals in the form of a pompon or in the form of children's balls made of corrugated paper [04]. They were obtained only last year at Ye notse University (Seoul, South Korea).
Electronic photos of graphene oxide pompons
According to the oxidation state, graphene oxides vary greatly and can contain from 3% to 40% oxygen by weight. The wide limits of the chemical composition (taking into account additional alloying atoms and groups) make it difficult to classify and standardize graphene oxides. Moreover, the composition can change not discretely, but continuously. However, for commercial needs, you can take the experience of classifications of natural diamonds, where the international classification consists of 3 thousand varieties that are absolutely understandable to professionals.
What is commercially significant is that in 2014 the basic prices of graphene and its oxides began to decline due to advances in industrial technologies and the expansion of production capacity in the world. About 50 producers of graphene and its derivatives compete fiercely, including in pricing.
Leading suppliers of Advanced Chemicals Suppliers (USA), Perpetuus Carbon (England), Graphenea (Spain).
Today, prices for high-quality aqueous emulsion of graphene oxide are at the level of $ 50 / g. In China, they offer products of variable quality for $ 20 / g. These prices are comparable to the price of platinum and some rare earth metals, which are widely used in modern technical devices. That is, graphene oxides have already overcome the price psychological barrier and can be used on an industrial scale.
In the U.S., National Nanomaterials has already launched the commercial product Graphenol, a family of functional graphenes, including graphene oxide ⬇️
Methods for obtaining graphene oxides
Four main methods for producing graphene oxide are known. All of them use the oxidation of graphite pieces in an aqueous environment of strong acids (for example, concentrated sulfuric acid) in the presence of highly active oxidants. These methods were followed by the names of Staudenmeier, Hoffmann, Brodie and Hammers. There are many varieties of them. Inventors strive to obtain stable quality, minimize rejection and reduce the cost of production. Thus, in 2014, vietnamese researcher Nguyen Heu Van proposed a two-stage method for producing graphene oxide without the use of strong oxidants - by anodic oxidation of graphite in sulfuric acid with microwave activation of the process.
For exotic forms, such as pompons, separate technologies are being developed.
Raw materials for graphene oxides are relatively cheap. Industrial devices made of corrosion-resistant alloys are expensive, but not crazy. The production infrastructure is obvious - on the basis of modern chemical plants. The problem is only in the technologies that the authors keep in the strictest confidence. Intellectual property contributes about 90% to the market value of modern goods based on graphene oxides. But for the foreseeable future, intellectual margins will disappear. Apparently, soon the cost of graphene oxides will approach the cost of foam and drywall.
First two-dimensional
Graphene oxide is the first two-dimensional material to reach the stage of commercial implementation. Figuratively speaking, it punches the way for other two-dimensional materials, such as phosphorene (phosphorus grid), silicene (silicon grid), silicatene (silica mesh), germanene (germanium grid), arsinene (arsenic grid), and two-dimensional polymers.
From nano- and microparticles of graphene oxide have already learned to make centimeter samples. So, recently Chinese scientists have developed a new material. It is so light that it is held on flower petals. The material consists of graphene oxide and lyophilized carbon. This spongy matter has a density of only 0.16 mg/cm3, making matter the lightest solid in the world [05]
Sample of spongy matter based on graphene oxide
In the report "Global Graphene Market (Product Type, Application, Geography) - Size, Share, Global Trends, Company Profiles, Demand, Insights, Analysis, Research, Report, Opportunities, Segmentation and Forecast, 2013-2020", the authors predict the growth of the market from $ 20 to $ 149 billion, or 44% per year.
Good and understandable growth, now let's look at the main shareholders and their well-being will be believed.
In the global market, the following corporations are leading in terms of activity: CVD Equipment Corporation, Graphene Nanochem PLC, Vorbrck Materials, XG Sciences, Haydale Limited, Graphenea, Graphene Laboratories, Bluestone Global Tech, Angstron Material, Inc., ACS Material, LLC.
Combining functional groups (hydroxyl, epoxyl, carbonyl, etc.), different graphene oxides allow for various types of interactions with biomolecules through electrostatic attraction, p-p stacking and hydrogen bonds.
Radionuclides are used in the sorption state on carriers of graphene oxide - these are short-lived alpha (213Bi, 225Ac), beta (90Y, 177Lu) or Auger - (67Ga) emitters.
Also sorption biosensors based on graphene oxide. They detect DNA in solutions. Graphene oxide can deliver absorbed oligonucleotides to living cells to detect biomolecules.
Finally, graphene oxides are able to accelerate the growth, differentiation, and proliferation of stem cells and therefore interfere with tissue engineering, regenerative medicine, and other biomedical fields. The safety of the practical application of graphene for biomedical purposes is almost not studied in practice.
An example of developments enshrined in an international patent are sorbents based on graphene oxides. Developed at the Department of Radiochemistry of the Faculty of Chemistry of Lomonosov Moscow State University (Russia).
The work was carried out in cooperation with the business of the United States. Sorbents can be used in a fundamentally new technology for cleaning liquids, for example, in nuclear power plants. Its main advantages are simplicity and high efficiency. In particular, during the sorption of uranium ions, graphene oxides are much superior to their closest analogues.

Technological scheme for obtaining pompons from graphene
The ultrasonic nozzle emits microdroplets of suspension consisting of nanoscale scales of graphene oxide. The drops enter a hot (160°C) reducing solution in an organic solvent. In the "hot bath" there is a reduction of graphene oxide. The scales of graphene then stick together in the form of a pompon.
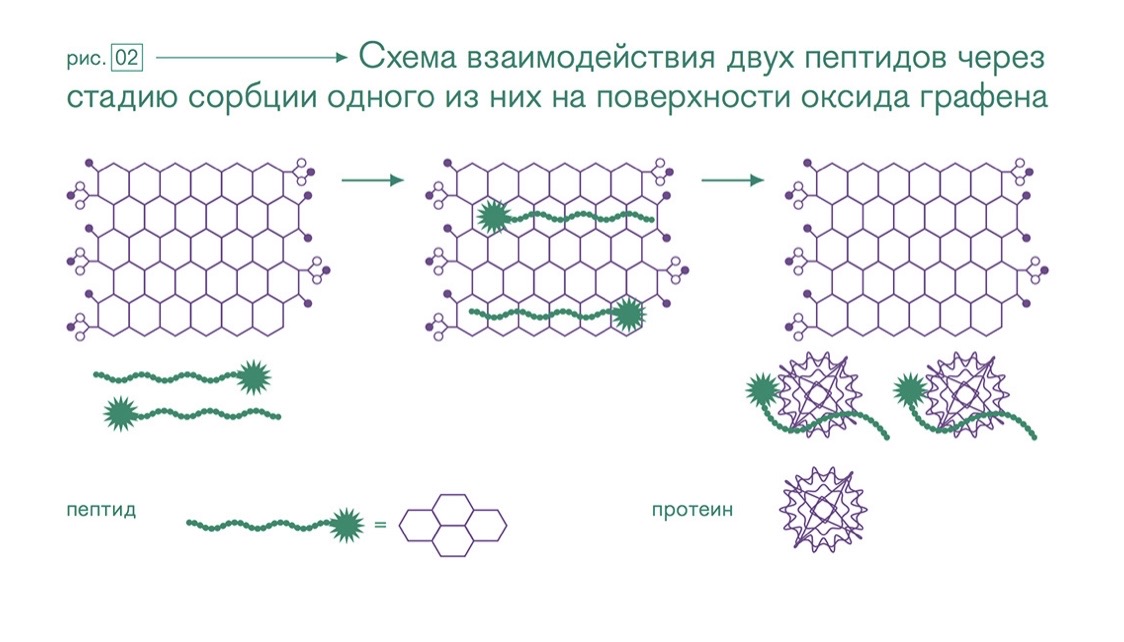
Scheme of interaction of two peptides through the sorption stage of one of them on the surface of graphene oxide
An example of the use of graphene oxides is a study by employees of Fuzhou University (Fuzhou University, China). The graphene oxide sorption system is an inexpensive method for determining protein-protein interactions. To develop peptide-based drugs, it is necessary to determine how the disease-related protein interacts with the peptides.
And for this it is necessary to be able to detect a signal indicating the interaction of proteins with peptides. Fluorescence resonance energy transfer (FRET) spectroscopy is usually used for this purpose.
Graphene oxide dampens the fluorescence of a peptide labeled with pyrene fragments (asterisk with a green chain) when the pyrene fragment converges with the carbon layer (the central fragment of the figure). However, when a protein binds to the peptide, the peptide breaks away from the graphene oxide layer and fluorescence resumes (pink asterisk on the right fragment).
The researchers tested their method on a peptide that is an indicator of HIV infection. A positive result was also found for a pair of peptide and protein - bungarotoxin, secreted from snake venom.








PS






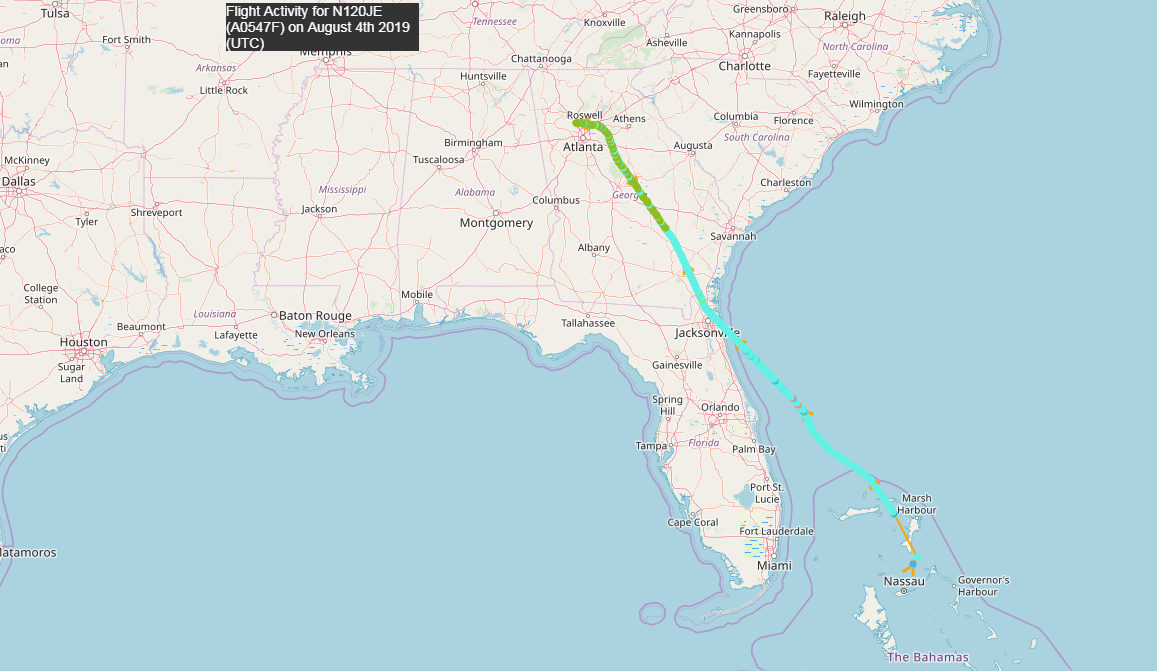
As you can see in the illustration above, on the flight-data service, where you can see the history of any registered aircraft, there is evidence that Epstein's plane continued to fly to the island where took place. As can be seen from the dates from the history of the aircraft, the board was active on August 4, 2019, August 1, July 27, July 23, July 17, 2019 and so on. On August 1 and 4, even before Epstein left the scene of the scandal, the board flew to Epstein Island:
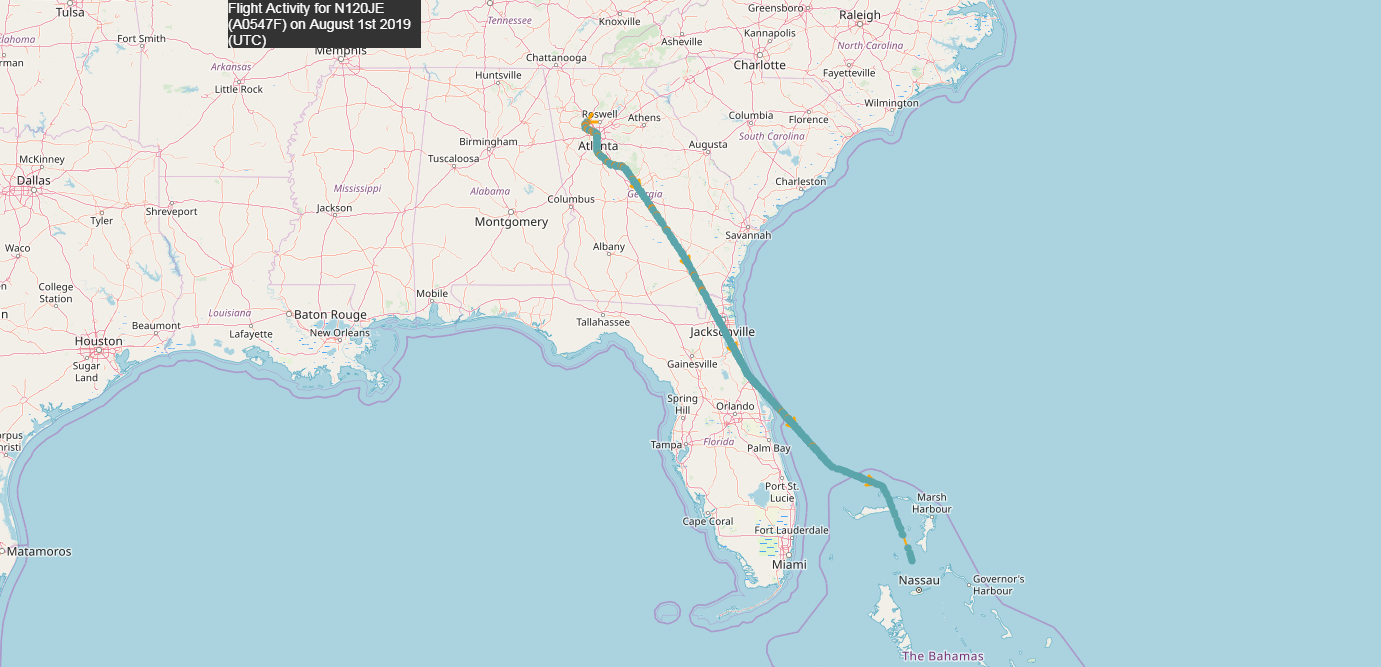
And why did Epstein's plane fly to Antarctica on July 23? "Lolita Express" in the area of the mysterious continent with landing on the Russian military airfield ?
July 23:
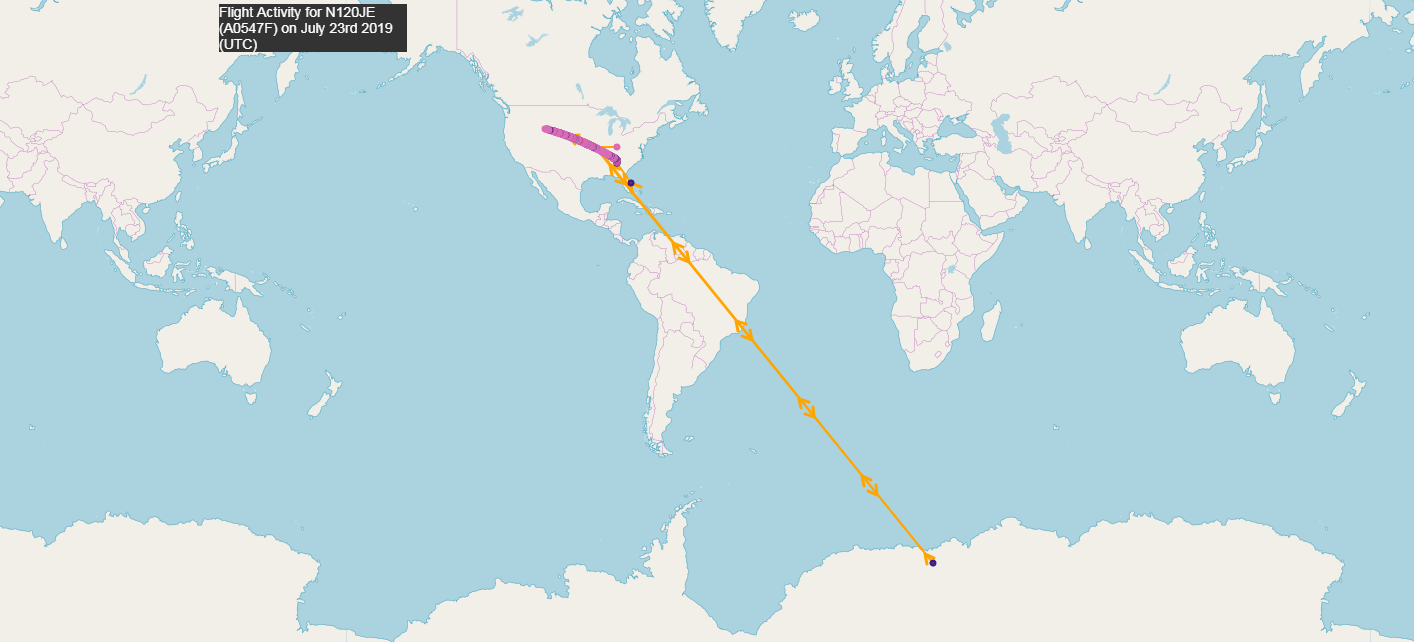
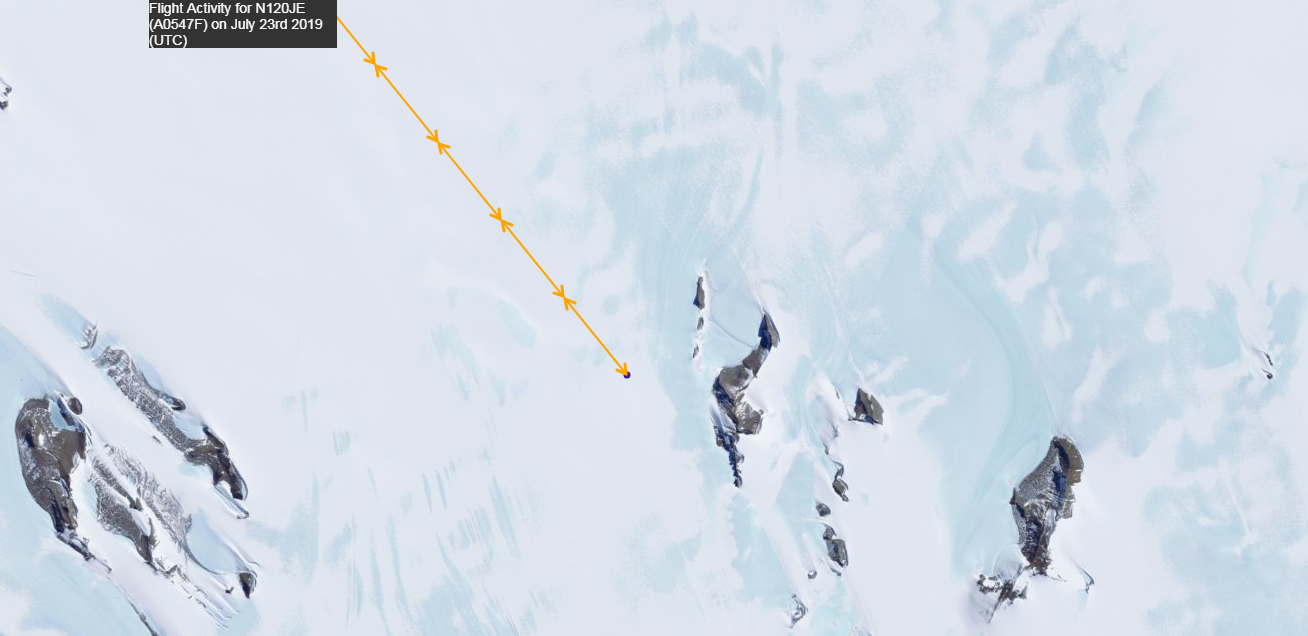

Why did the plane fly so far ✈️
There are none of the 22 airports that can serve airplanes (helicopters only). There is only the nearest runway near the visible landing pad. This is the Russian - Perseus Airfield (3 km ice), the runway closest to the Princess Elizabeth station, which has a length of 1420 m. Although, maybe there is something else nearby, capable of receiving aircraft of this class.
Fucking pidaras, we will tear you into two parts from the legs where they diverge and to your filthy 👄














Financing of works on graphene oxide in the Russian Federation is carried out by dozens of grants in several areas, for example, grants of the President of the Russian Federation MK-7155.2013.3 and MK-5847.2014.3; RFBR N12-03-00533, 12-03-00615, 14-23-01015 and 14-29-04071; within the framework of the Program of Fundamental Research of the OCHNM RAS N OH2.4; grants from Rusnano (MSU-06/1 agreement) and the UMNIK program, etc.



https://telegra.ph/STATE-BIOTERRORISM-CROWN-08-09
https://telegra.ph/STATE-INFORMATION--BIOLOGAL-AGRICULTURAL-TERRORISTS-OF-THE-USA-08-29
Let's go General, We're ready!





Ааааааааааааааа 😂 Ааааааааааааааааа

- - Y᧐ᥙr 𑀝rᥱɗ᧐ ?
@brigadegeneralino_bot
- Aᥣᥕᥲys 🇺🇸
𐌑r. 𑀝᧐ᥣ᧐ᥒᥱᥣ