PCR - A critical examination
translated by Corona Investigativeby Raphael Haumann
PCR, which stands for polymerase chain reaction, is first of all a molecular biological technique for making DNA copies. It is based on the possibility of artificially doubling DNA strands by means of certain enzymes known as polymerases. A technology that was developed in the mid-eighties. With this technique, theoretically from only one single DNA strand after only thirty-two duplication steps, about four billion equal strands are obtained. Each of these doublings is called a cycle. After a fixed number of cycles, the amount of DNA produced can be determined and thus the tiny initial amount of DNA can be calculated back to the original amount that could otherwise not have been determined diagnostically. PCR tests of any kind are highly sensitive and depend on many parameters, making them extremely error-prone. Due to the exponential growth of DNA, even a small change in the initial conditions or a small deviation from the usual process has a great impact on the test result. In general, PCR replicates one or more relatively short DNA sequences. Not complete genomes.
For example, the well-known test by Christian Drosten, searches for a sequence of 213 nucleotides in an alleged viral genome of 30,000 nucleotides in total. In other words, less than one percent.
Each laboratory can select any gene sequence. Some laboratories search for 2 or 3 sequences simultaneously. Each laboratory decides for itself whether to report a positive result when one of two or two of three sequences are found.
The whole thing is like looking for a certain animal in a huge zoo. Let's say a jaguar and each researcher pays attention to a different characteristic. One looks for fangs, one looks for spotted fur, one looks for quiet paws and one looks for fangs and reflective eyes. Everyone will find if he only looks long enough. Whether however, all researchers found the same animal remains uncertain. The searched gene sequences can be only parts of genetic fragments, or belong to the microvesicles produced by the body billions of times.

Furthermore, a PCR test says absolutely nothing about the significance of the RNA being searched for. Therefore one thing is clear from the beginning. With a PCR test you can not detect a virus or any kind of infection. In the best case one can only say that the short gene sequence is present in a sample or not. However, whether this is of importance for the health of the person tested is a completely different matter and will not be discussed in detail in this article.
Three main components are used in the polymerase chain reaction.
- Polymerase
- Primers
- Nucleoside triphosphates - short dNTPs, i.e. the building blocks from which DNA is constructed.
These components are given a solution called "buffer", which contains magnesium ions. The content of magnesium has to be adjusted to the chosen polymerase and can influence the result.
POLYMERASE
The heart of the PCR is the DNA polymerase. An enzyme which can copy DNA sequences. Only polymerase from heat-loving organisms is used for PCR, because PCR requires relatively high temperatures to cleave the double-stranded DNA. There are many different polymerases which can be used. They differ mainly in five properties.
- Thermostability, i.e. how stable they are at higher temperatures.
- Processiveness. This is a measure of how many nucleotides a polymerase can attach before it separates from the template DNA. Different polymerases have different affinities for different DNA sequences.
- Speed. A high degree of processing as well as a high resistance to inhibitors, for example, leads to higher speeds in DNA synthesis.
- Accuracy. Some polymerases are more prone to incorporate wrong nucleotides than others. There are also those that contain some kind of error correction, i.e. they detect and remove wrongly placed nucleotides.
- Specificity. One of the many problems with PCR is low specificity. Not only is the DNA being searched for amplified, but also many other sequences that falsify the result. The specificity varies from polymerase to polymerase.
PRIMER
PCR does not amplify an entire DNA sequence but only a small section, typically about two hundred to three hundred nucleotides. The start and end point of the sequence range to be amplified is determined by special molecules called "primers". Primers are short single-stranded DNA sequences, typically between fifteen and thirty bases long, which attach themselves to specific sites on the target DNA. Primers also have several persistent problems. The biggest one is the following. During the initial mixing of the components, reactions already begin. Primers can dock quite unspecifically at room temperature at any position of the template DNA or even to each other. Wrongly primed strands are duplicated by the polymerase which blindly copies everything a primer has. One tries to get a grip on these problems by modifying the polymerase so that they are inactive at room temperature and only start working at high temperatures. This is called "Hotstart PCR". But because these special polymerases are more expensive than the simple ones, this procedure is not used in all PCR tests.
THERMOCYCLER
Of particular importance in PCR is the most accurate and consistent temperature management possible. When PCR was started in the mid-1980s, the test tubes were still placed by hand in water baths at different temperatures and the different reactions were run. Since 1988 there are devices that do this, so-called thermocyclers. Today, there are many different models, cheap and expensive of very different quality. The reliability of these devices is absolutely crucial. Which is why they require continuous monitoring.

A study of Korean scientists (1) came to the following conclusion regarding the reliability of thermocyclers.
"The performance of thermal cyclers for polymerase chain reactions (PCR) is of great concern in terms of the reliability of PCR-based assays, particularly when rapid cycling conditions are applied to small volume reactions. In this work, the precision of the temperature controls during rapid thermal cycling was measured in 19 commercial thermal cyclers of 8 different models. Under the given conditions, a majority of the tested instruments showed prominent curving, undershooting, and/or overshooting in their temperature profiles, which substantially influenced the results of the temperature-sensitive multiplex PCR. [...]Only 2 of the 19 tested instruments showed nearly ideal behavior. [...] It is strongly hoped that these problems will be addressed by manufacturers and that they will make substantial improvements in the precision and efficiency of thermal cyclers."
The thermal cyclers thus represent another major problem area in the field of PCR.
ONE TEST?
A frequently recurring question is whether there are actually only one or several different PCR tests. As you can see from the above, no two tests can be compared with each other due to the many possibilities of choice and the many sources of error. For example, the US Food and Drug Administration (FDA) lists 178 different commercially available Covid-19 PCR test kits from which US laboratories can choose (2). Each test kit differs from the others in many important ways, such as the reagents selected or the gene sequence sought.
MANUFACTURER'S DATA
In PCR, huge differences in the results are possible. Not only with different test procedures, but even with the same test procedure, since there are many parameters that can be changed, knowingly or unknowingly, and an enormous number of sources of error lurk. This is known to all molecular biologists who work with it. Because of the resulting low reliability, PCR is not a procedure that can be used for medical diagnostic purposes. The manufacturers of the individual components explicitly point this out. According to their own statements, the world's largest supplier of PCR components is Thermo Fisher, a US company with seventy thousand employees. On the website of this company you will find a huge selection of components for all kinds of PCR methods. At the bottom of each page, in small print, you will find the following note (3):
"For Research Use Only. Not for use in diagnostic procedures."
This could be an exception. So let's have a look at other providers websites. QIAGEN, a Dutch company with headquarters in Duesseldorf, Germany, adds the following sentence to its PCR products:
"This product is not intended for the diagnosis, prevention, or treatment of a disease."
At AGILANT, a U.S. company with fifteen thousand employees, under each component is written (4):
"For Research Use Only. Not for use in diagnostic procedures."
A search for DNA polymerases that can be used for diagnostics is the first result that Japanese company TAKARA BIO comes up with. This company offers special polymerases, which should also be applicable for diagnostic purposes. However, explicit reference is made to the following (5):
"This product is a general purpose reagent intended . Outside of the United States, this product is intended for research use only unless otherwise stated. This product is not intended for a specific application or made for any particular clinical use. The performance characteristics of this product have not been fully established. It is the user’s responsibility to validate the performance of the product, and any component thereof, for any particular use."
You can find it like this or similar with all manufacturers. Either it is clearly stated that the components are not intended for diagnostic use, or if they are sold for such use, it is stated that adaptation to the prevailing laboratory conditions is essential and the purchaser alone is responsible for the validation of the purchased components for the intended use. It is also noted that the performance characteristics are not fully known. From such formulations it is again clearly evident that there is obviously a high degree of uncertainty in PCR with regard to the results.
THE DROSTEN TEST
To conclude this chapter, we will look at the famous PCR test published by Christian Drosten just days after the first alleged Covid-19 cases in China, which the WHO uses as an additional safeguard for its own test procedure. The publication "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR" (6) states that Drosten used a ready-made commercial kit, with the name "Superscript III one step RT-PCR system with Platinum Taq Polymerase (Invitrogen)".
We also learn from the publication that the other laboratories that claimed to have verified his test used the following kit "TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher)".
Let's have a look at both products. We can see on the website (7) that the manufacturer provides the commercial Master Mix, which Drosten used, only for research, not for diagnostic purposes. It is clearly mentioned on the website as well as in the package insert to the product:
"For Research Use Only. Not for use in diagnostic procedures."
The situation is the same for the master mix (8)used by the other participating laboratories . This product is also not suitable for diagnostic purposes as can be seen on the website and on the package insert.
"For Research Use Only. Not for use in diagnostic procedures."
FURTHER ERROR SOURCES
So much for the PCR itself. The diagnostic tests that are made with it do not only insist on the PCR procedure. For a test, a sample must first be obtained. There are different ways to perform a swab. There are also big sources of error. For example, a study by Chinese researchers in May 2020 (9) reports that the number of CoVID-19 PCR tests evaluated as positive fluctuates greatly depending on the variant of the sample taken. In the case of bronchoalveolar lavage, i.e. the collection of mucus from the lungs, the rate of tests interpreted as positive was 93 percent. If sputum, i.e. mucus from the throat, was used, the rate was 72 percent. For swabs from the nose, 63 percent and for revenge swabs only 32 percent. The number of results that can be interpreted as positive depends on how the sample is collected.
Contamination of the specimen by the collector or the environment is also a problem. How and how long the sample is kept until it is tested is also important.
Other sources of error can arise during the preparation of the samples in the laboratory. There, the RNA must be extracted from the sample and converted into DNA, since PCR only works with DNA. There are also specially compiled commercial kits for the extraction of RNA using very different methods. Thermo Fisher lists four different ways to extract RNA for PCR tests (10). The manufacturer also directly points out that there is a problem with contamination by the body's own so-called genomic DNA, which can be minimized but not eliminated. Such contamination can lead to false positive results. There are also many parameters to consider when converting RNA into DNA, and there are dozens of products to choose from, all of which have different properties and can therefore contribute to very different initial conditions. The efficiency of RNA conversion to DNA is typically somewhere between 5 and 50%.
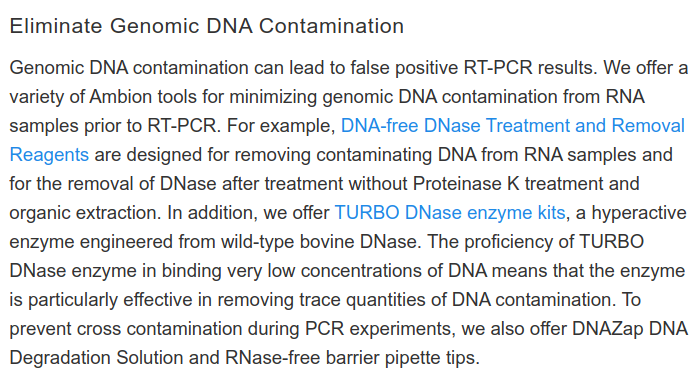
What should this long-term view show? It should make it clear that there is no such thing as the "one" PCR test. Even when using absolutely the same components, in the same laboratory, by the same employees, it is difficult, almost impossible, to obtain reproducible results.
In a study (11) from January 2020, three researchers tested 18 patients with pneumonia daily by PCR tests for the allegedly dangerous RNA sequence. Data points on the blue cut-off line are considered negative. All points above this line are considered positive. You can see how the results for many patients fluctuate greatly. Sometimes there are results that are considered positive, sometimes results that are considered negative. This often varies from day to day. Trust in the validity of the tests is certainly not given.
POSITIVE OR NEGATIVE?
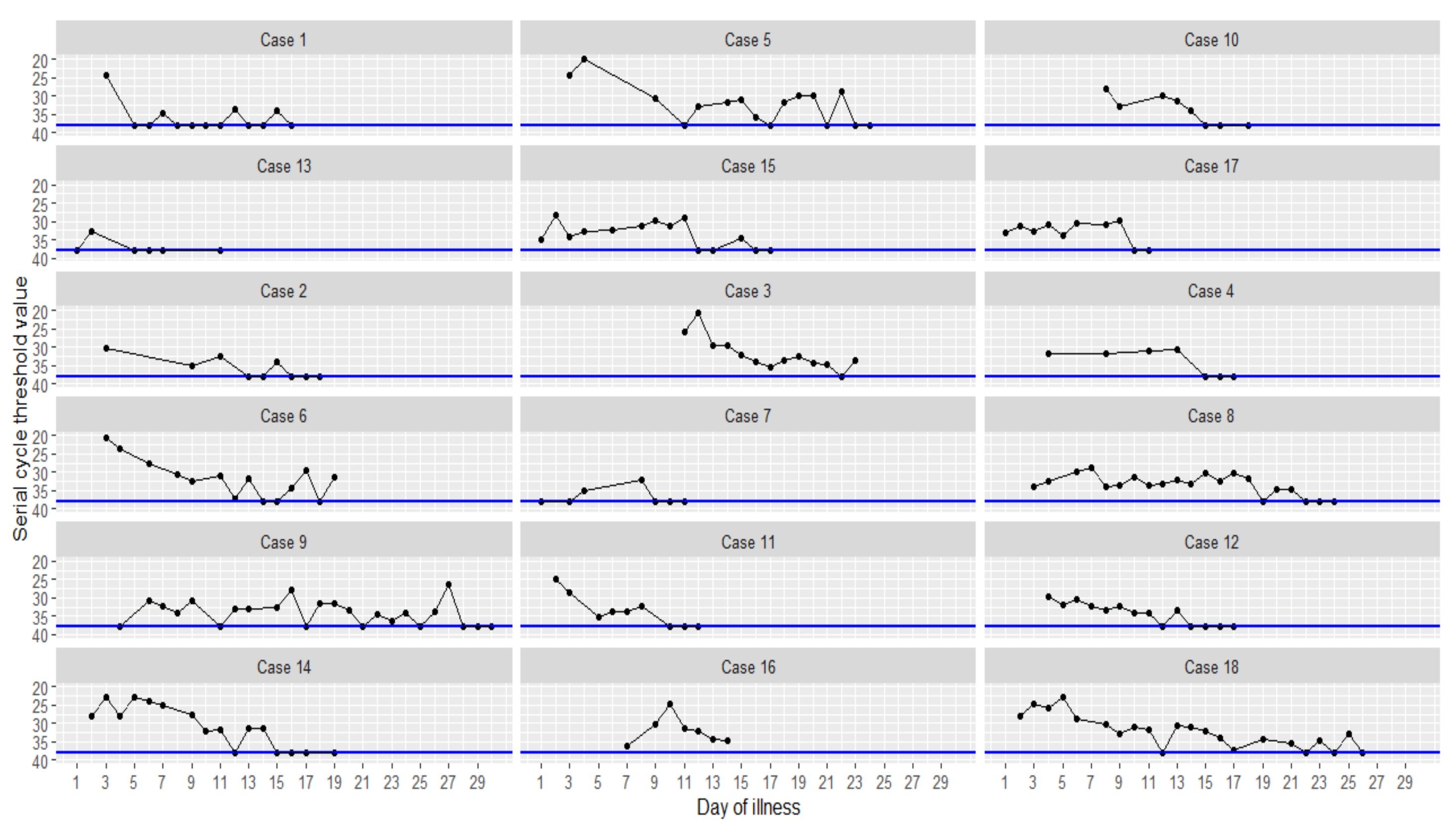
So far we have seen that reliability and reproducibility are very poor due to the complexity and multiple sources of error. Basically, each PCR test is unique and cannot be compared to any other. Another problem with the use of these tests in diagnostics is the fact that they are not binary. They do not give a yes or no result, no positive or negative. In order to assess a result, the luminescent genetic material, which is made visible with fluorescent molecules, is examined. It is important to note how long the test has been run, i.e. how many cycles have been made. The manufacturer, Thermo Fisher, explains the procedure on his website (12):
"PCR steps of denaturation, annealing, and extension are repeated (or “cycled”) many times to amplify the target DNA. The number of cycles is usually carried out 25–35 times but may vary upon the amount of DNA input and the desired yield of PCR product. If the DNA input is fewer than 10 copies, up to 40 cycles may be required to produce a sufficient yield. More than 45 cycles is not recommended as nonspecific bands start to appear with higher numbers of cycles. Also, accumulation of by-products and depletion of reaction components drastically lower PCR efficiency, resulting in a characteristic plateau phase for a PCR amplification curve (Figure 7). Conversely, low cycle numbers are preferable for unbiased amplificationand accurate replication of target DNA."
The amplification of the DNA must therefore be stopped at some point, otherwise the polymerases would simply continue to form further DNA until no more nucleodite is present in the solution, whereby more and more unwanted by-products accumulate. If you do not do this or set it too high, there will be many tests that are incorrectly considered positive. This is of course a very big problem if you want to use such a test for diagnostics. A substantial number of tests can be considered false positive if the PCR is performed too long. But the matter becomes even more problematic. Every laboratory can set this cut-off as they wish. There are no guidelines. This means that laboratories can manipulate the number of positive tests simply by deciding on the number of cycles at which they set the cut-off. On this subject, let us listen to the world's leading expert in the field of quantitative PCR tests, the British molecular biologist Professor Dr. Stephen Bustin, who has published several textbooks on this topic. In an online radio interview on 04/14/2020 (13) conducted by David Crowe from "The Infection Myth" website he said the following:
David Crowe: So what I have seen with the corona virus testing is that they choose a cycle number, I have seen 36 and 37, I haven't seen it published very much, and if you obtain sufficient DNA by that cycle than it is considered positiv and if you don't it is considered negative. That seems a kind of arbitrary.
Dr. Stephen Bustin: Yes that is absolut nonsense. (laughs) It makes no sense, It makes no sense whatsoever. So let me go back to the cycle number. It depends on a lot of different things. First different instruments give you different cycle numbers. Different PCR Master Mixes can gives you different cycle numbers. Different lots of probes can give you different cycle numbers. So the cycle number per se is not a good measure. The second point is that for most instruments once you get above a cycle of about 35, then you start worrying about the reliability of your result, because that would be roughly equivalent to a single initial DNA sequence. What you want to do, you want to be certain that the result you get are in the 20th or 30th.
David Crowe: If you cycle to many times, can you start to get like a ghost production of DNA?
Dr. Stephen Bustin: Yes. Again it depends how your PCR test is structured. In principal using a probe you shouldn't. But in practical of course you might. Yes.
David Crowe: Yes and so if you where to go to, say 40 cycles, you might get a positive result that might be a false positive and you PCR is just started to string basis together.
Dr. Stephen Bustin: I would be very unhappy about a 40 cycle piece.
David Crowe: There is, I do not know if you know this, but there is British recommendation for Corona virus testing, that seems to indicate, that every part of England can do what they like in terms of choosing a cycle number and they say if you cycle number is over 40, then it needs to go for further testing. But I was surprised that anybody would do that.
Dr. Stephen Bustin: No. I think that the number of cycles by itself, and again we published this, the number of cycles is quiet meaningless. There are other parameters that you can define before the number of cycles mean anything.
David Crowe: Again. In the two papers I have seen where the number of cycles used was published, once 36 was set as the cut-off for a positive result, and then 37 to 39 cycles were considered an undetermined result, which required further testing. In the other paper, 37 cycles were taken as the cut-off without a possible uncertain result.
Dr. Stephen Bustin: I would be very unhappy about that. The result would be totally dependent on the device, the reagents and the probe, and also dependent on the procedure.
High numbers of cycles, which are absolutely normal for a corona test, regularly lead to false positive test results, which depend solely on the way the test is performed. This has been known since the invention of PCR tests. Some governments recommended a cut-off to their laboratories. The Chinese government, for example, was guided by the work with the 18 patients who were once considered positive to be negative. The researchers set the cut-off at 37 cycles, which is quite high. Other countries also have varying requirements, but they all generally allow for high cycle counts. If high cycle numbers and all the possible sources of error can lead to false positive results, then we need to take a closer look.
FALSE-POSITIVE
There is no molecular biological test that is 100 percent accurate. Medical tests are usually evaluated by three important parameters. Sensitivity, specificity and PPV (positive predictive value). Sensitivity represents the reliability with which positive ones are actually detected as positive. The specificity, on the other hand, indicates the probability that negative values are actually recognized as negative.
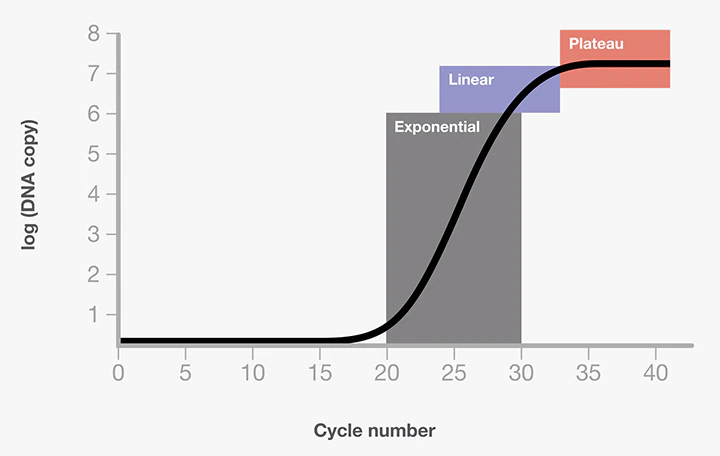
For example, at a specificity of ninety-five percent, five percent will be falsely recognized as positive. The PPV indicates the probability that a positive result is a true positive and not a false positive. This requires knowledge about the actual level of the so-called " infestation " in the population. However, the infestation of Covid-19 can only be determined on the basis of the tests, which means that we are dealing with a vicious circle and that in the end there are only estimates of the PPV. Let us take an example to illustrate this. In a population of one hundred people, one is actually positive. That is, a contamination of one percent. The test used has a sensitivity of 100 percent and a specificity of 95 percent. If all one hundred people are tested, there are six positive results. One true positive and 5 false positives. The PPV of this test would be only 17 percent. Although at first glance the test appears to be accurate at 100 percent and 95 percent, there is only a 17 percent chance that someone who tests positive is actually positive. The significance of a test depends very much on the level of infestation. If we choose two percent instead of one percent, the PPV will improve from 17 to 29 percent while the other values remain the same.
If many tests are taken and there is little or no infection, then there are relatively many false positives and the reliability of the test suffers greatly. For this reason, mass testing is dangerous. If you then use a test based on PCR, which is extremely error-prone and unreliable, and thus test millions of people randomly, it is inevitable that many people will test positive by mistake. This can create the impression of a pandemic, where in reality nothing is happening. Even the US Food and Drug Administration (FDA) reports the following on their website (15):
"Because all test will return some false positive and some false negative results, broad use of the tests, when not appropriately informed by other relevant informations, such as clinical history or diagnostic test results, could identify too many false-positive individuals."
So this is a well known problem. On the other hand, it is noticeable that false positive results hardly play a role in the public debate. It is pretended that they do not exist. This of course facilitates the collection of fictitious numbers of so-called "infected" people. Any PCR result that is interpreted as positive can be taken as genuinely positive. A good example is the work published by Christian Drosten et altera in mid-January (16). The Drosten test gives a sensitivity of 95 percent and a specificity of 100 percent. So no false positive results. Of course, it has been known for a long time that such research results in no way reflect everyday diagnostic practice. The paper "Interpreting a covid-19 test result" (17) states in regard to this:
"The reported accuracies are much higher for in vitro studies that measure the performance of primers in coronavirus cell cultures under precisely controlled conditions."
Some Chinese researchers described the results of their research (18) on PCR test error rates in early March 2020 as follows:
"Results: When the infection rate of the close contacts and the sensitivity and specificity of reported results were taken as the point estimates, the positive predictive value of the active screening was only 19.67%, in contrast, the false positive rate of positive results was 80.33% Conclusion: In the close contact of COVID-19 patients, nearly half or even more of the 'asymptomatic infected individuals' reported in the active nucleic acid test screening might be false positives."
Here, a very high rate of false positive results was detected. This work was cited by a number of critics of the PCR tests around mid-March. Pub Med, one of the leading internet sites publishing medical studies, decided to withdraw this work. In the statement of reasons it says:
"The article 'Potential false-positive rate among the 'asymptomatic infected individuals' in close contact of COVID-19 patients" was under strong discussion after pre-published. Questions from the readers mainly focused on the article's result...........the article was decided to be offline by the editorial board from the pre-publish lists."
This is a case of censoring unpopular scientific work at a time, in the middle of march 2020, when there was massive censorship worldwide with regard to criticism of testing, scientific foundations and measures.
DARTMOUTH CASE STUDY
In 2006, Dartmouth medical center, a major hospital in New Hampshire, experienced mass panic due to suspected whooping cough. The New York Times published an article about it a year later (19). The title alone shows how topical it is. "Faith in Quick Test leads to Epidemic That Wasn't". In mid-April 2006, a doctor at the hospital began coughing heavily and continuously. This went on for two weeks. This was followed by a week of sporadic coughing. A colleague of hers, a specialist in infectious diseases, suspected it might be whooping cough. On closer inspection, she noticed that some other employees were also coughing. The specialist then sounded the alarm, fearing a pertussis epidemic. The panic spread quickly and a PCR was quickly performed on nearly 1000 hospital employees. A positive result was reported for 142 employees. As a result, thousands of people were prophylactically treated with antibiotics and vaccinated. About 8 months later, after a proper laboratory test for pertussis was performed, it was discovered that the whole thing was a false alarm. Not a single case of pertussis could be detected. Normal cold symptoms were thought to be pertussis in the panic and due to the highly erroneous PCR test. At this point I would like to quote some passages from the article, because it is very eye-opening.
"Now, as they look back on the episode, epidemiologists and infectious disease specialists say the problem was that they placed too much faith in a quick and highly sensitive molecular test that led them astray.
Infectious disease experts say such tests are coming into increasing use and may be the only way to get a quick answer in diagnosing diseases like whooping cough, Legionnaire’s, bird flu, tuberculosis and SARS, and deciding whether an epidemic is under way.
There are no national data on pseudo-epidemics caused by an overreliance on such molecular tests, said Dr. Trish M. Perl, an epidemiologist at Johns Hopkins and past president of the Society of Health Care Epidemiologists of America. But, she said, pseudo-epidemics happen all the time. The Dartmouth case may have been one the largest, but it was by no means an exception, she said.
There was a similar whooping cough scare at Children’s Hospital in Boston last fall that involved 36 adults and 2 children. Definitive tests, though, did not find pertussis.
“It’s a problem; we know it’s a problem,” Dr. Perl said. “My guess is that what happened at Dartmouth is going to become more common.”
Many of the new molecular tests are quick but technically demanding, and each laboratory may do them in its own way. These tests, called “home brews,” are not commercially available, and there are no good estimates of their error rates. But their very sensitivity makes false positives likely, and when hundreds or thousands of people are tested, as occurred at Dartmouth, false positives can make it seem like there is an epidemic.
Yet, epidemiologists say, one of the most troubling aspects of the pseudo-epidemic is that all the decisions seemed so sensible at the time.
“Almost everything about the clinical presentation of pertussis, especially early pertussis, is not very specific,” Dr. Kirkland said.
That was the first problem in deciding whether there was an epidemic at Dartmouth.
The second was with PCR., the quick test to diagnose the disease, Dr. Kretsinger said.
With pertussis, she said, “there are probably 100 different PCR protocols and methods being used throughout the country,” and it is unclear how often any of them are accurate. “We have had a number of outbreaks where we believe that despite the presence of PCR-positive results, the disease was not pertussis,” Dr. Kretsinger added.
“Because we had cases we thought were pertussis and because we had vulnerable patients at the hospital, we lowered our threshold,” she said. Anyone who had a cough got a PCR. test, and so did anyone with a runny nose who worked with high-risk patients like infants.
....
Dr. Cathy A. Petti, an infectious disease specialist at the University of Utah, said the story had one clear lesson.
“The big message is that every lab is vulnerable to having false positives,” Dr. Petti said. “No single test result is absolute and that is even more important with a test result based on PCR”"
Doesn't all this seem awfully familiar to us? That Dartmouth was a mere mass panic could be found out afterwards, because whooping cough is correlated with the pertussis bacterium and this can be detected not only in a PCR test, but also in a complex laboratory process in pure culture. So there is a gold standard, a test that has been tried and tested for decades and which reliably detects the presence of a correlated bacterium, and this test subsequently turned out negative in every test. An infestation of 0 percent. A false positive rate of 100% of positive results of PCR tests. Here, the PCR tests had created an epidemic that did not exist.
SENSITIVITY ≠ SENSITIVITY SPECIFICITY ≠ SPECIFICITY
When it comes to the concepts of sensitivity and specificity, one must always see them in context. Under real clinical conditions, you need a gold standard, which has been introduced by methods that are beyond any doubt. For example, whether a woman is pregnant can be reliably detected with an ultrasound scan from about the sixth week of pregnancy. This can serve as a gold standard by which other pregnancy tests can be evaluated. The sensitivity and specificity of a new test must be compared with such extremely reliable diagnostic methods to get a good impression of the quality of new tests. But there is no gold standard for the alleged corona viruses. There is no way to reliably detect so-called viruses. This raises the question of where the numbers actually come from. Dr. Sanjaya Senanyake an Australian expert on infectious diseases gives the following insight in an interview with ABC TV (20):
Reporter: "Now for over an week we have putting your questions to an expert panel. This week we are joined by our national medical reporter Sophie Scott and Dr. Sanjaya Senanayake an infectious disease specialist at the Australian National University. Dr. Senanyake a question received by the ABC News website. How accurate is the current testing?"
Dr. Senanyake: "That is a hard one to know Jeremey, because there is no goldstandard to really compare this to. So if we had a new test to detect [the bacterium] staphylococci in the blood, we would already have blood cultures, which is our gold standard that we have been using for decades, and we could match this new test against that. But for COVID-19 we don't have a gold standard test. So the current test we are using, the PCR Test, where we put a swap in the throat and in the back of the nose their are our gold standard, but trying to work around that we think that it is probably picking up about 70% of cases."
Oha! The PCR test is supposed to be its own gold standard and one thinks the sensitivity is what? 70 percent! And even this value is just out of the air. A pure estimation without any scientific basis. If you leave the real-world diagnostic situation and go into a highly artificial research situation, sensitivity and especially specificity can be determined more easily, because you can use samples that you know with a high degree of certainty what they contain. If a Christian Drosten in his work published in mid-January states a sensitivity of ninety-five percent and a specificity of one hundred percent, i.e. he does not want to have achieved false positive results, then this must be understood in the context of a research situation. He knew in advance which sample contained what and so, given the many possibilities to deceive oneself self-confidently or unconsciously, it is not surprising that he considers his test to be extremely accurate. In diagnostics, the situation is completely different, and one therefore speaks of the analytical sensitivity and specificity of the research situation. It is also referred to as clinical sensitivity and specificity, which means that these values are not derived from highly artificial research, but were determined under real diagnostic circumstances, and the lack of a gold standard plays a significant role here.
Let us consider a scientific paper published on May 12, 2020 (21) by three British researchers, a doctor, an associate professor of clinical epidemiology and a professor of internal medicine. We read:
"No test gives a 100% accurate result; tests need to be evaluated to determine their sensitivity and specificity, ideally by comparison with a “gold standard.” The lack of such a clear-cut “gold-standard” for covid-19 testing makes evaluation of test accuracy challenging."
How are the correct parameters determined in the so-called COVID-19 tests? This is what it says:
"A systematic review of the accuracy of covid-19 tests reported false negative rates of between 2% and 29% (equating to sensitivity of 71-98%), based on negative RT-PCR tests which were positive on repeat testing."
This must be repeated. The sensitivity of the tests is determined by whether, in the case of a first test interpreted as negative, a subsequent test is interpreted as positive. The test whose parameters are unknown is used to determine the parameters. Of course you cannot solve an equation if you only have unknown parameters. Therefore it is simply assumed that a result interpreted as positive is practically beyond any doubt. This is what the paper says:
"In the case of the nasopharyngeal swab RNA test for covid-19, the positive likelihood ratio is about 14, which is excellent. A positive covid-19 test result should be very compelling."
A reassuring statement. But how do the authors prove it? As reference for this statement they refer to the following work "False-negative results of initial RT-PCR assays for COVID-19: A systematic review" (22).
The authors of this study simply looked through five existing studies and searched for the reported sensitivity. No mention is made of the specificity of PCR tests. It simply says casually:
"Cases with negative RT-PCR resulta at initial testing and found to be positive in a subsequent test are commonly considered cases with an initial false-negative diagnosis."
And in other places it says to positive results:
"No studies reported criteria for positivity."
The authors do not lose a single word about specificity throughout the article. The given work supports the statements of a positive interpreted result is extremely robust. So not at all. It is simply believed that the specificity is very high. Up to the absurd estimation of Christian Drosten, his test does not deliver false positive results. In practice, sensitivity is therefore determined by testing the same sample several times. If a test has a negative result first, then a positive result, it is simply assumed that the positive result is correct and the negative must be false. Thus, sensitivity suffers, which is related to the rate of false negatives. A hundred percent specificity is achieved by simply assuming that if there are contradictory results, the test interpreted as positive is correct. This is really frighteningly bad scientific practice. It is not only assumed that an unknown quantity is known, but also that this quantity is actually 100 percent. That there are no false positive results. This is completely unjustified in view of the enormous error-proneness of PCR tests. This is all based on faith alone.
It appears that both clinical sensitivity and specificity are unknown for all applied corona PCR tests. As there is no gold standard against which these tests can be measured. With the hysteria slowly subsiding in mid-October and more people coming to their senses, it is not surprising that critical works are emerging. On September 28, 2020, three researchers reported (23) the following false positive rates.
"Contrary to the practice during previous epidemics, with COVID-19 health authorities have treated a single positive result from a PCR-based test as confirmation of infection, irrespective of signs, symptoms and exposure. This is based on a widespread belief that positive results in these tests are highly reliable. However, evidence from external quality assessments and real-world data indicate enough a high enough false positive rate to make positive results highly unreliable over a broad range of scenarios."
"The high specificities (usually 100%) reported in PCR-based tests for SARS-CoV-2 infection do not represent the real-world use of these tests, where contamination and human error produce significant rates of false positives."
"Widespread lack of awareness of the real-world false positive rates affects an array of clinical, case management and health policy decisions. Similarly, health authorities guidance on interpreting test results is often wrong."
And in the meantime, a few experts are finally making their voices heard in the mass media. For example, Dr. Mike Yeadon, former leading scientific consultant and vice-president of the pharmaceutical company pfizer, made a statement in mid-September:
Julia Hartley-Brewer: We saw yesterday 3991 positive cases for Corona Virus. It's up 50% in a week. You can understand why huge sums of people are incredibly worried that we are seeing that uptake in cases. Should we be worried?
Dr. Mike Yeadon: Thanks for having me back Julia. So, of course I definitely don't want to dismiss the possibility that these numbers are real and that people will get ill and potentially get to hospital or even die. But the evidence to date I think suggests, that all or substantial part of these positives could be due to what's called false positive tests. Now, governments knows that the PCR tests are very sensitive and all tests have a propensity to throw up a particular false positive rate. What's really frustrating Julia, and your listener should know this, the government either doesn't know what the false positive rate is, or if it does, it is not declaring it and continuing with the assumptions that it is zero. And it is definitely not zero. Prof. Carl Heneghan (Director of the Centre for Evidence-based Medicine at Oxford University) did a calculation and he showed that if the false positive rate is as little as 0.1% then more then half the positive tests are in fact false or fake. And I think the false positive rate is probably much higher, possibly 1%. And if that's true, most or all of the positives are actually false and they are not infectious or ill.
Although it is generally assumed that PCR tests are 100% specific, this is certainly not the case as we have seen. The clinical specificity of the test is largely unknown, but there is some evidence. For example, a German study in April 2020 (24), in which samples that certainly did not contain the sought-after RNA were sent to a total of 463 laboratories, came to the conclusion that a total of 1.9 percent of the reported test results were false positive. Now let's take a quick look at what this could mean in terms of numbers.
Since it' s about specificity, not sensitivity, we simply set the latter to 100 percent. For the specificity we choose 98.1 percent, as the German studies suggest. If we now choose a 0 percent screening, i.e. the complete absence of any real new disease, and even of the RNA sequence itself, and enter the number of tests performed in Germany by mid-October 2020, namely 18 million, then theoretically we can expect 342,000 false positive results. According to the German Epidemic Control Agency RKI there were 330,000 cases in Germany in mid-October, so what Dr. Yeadon and others are now saying may well be true. The majority if not all positive results of the PCR tests could be false positive.
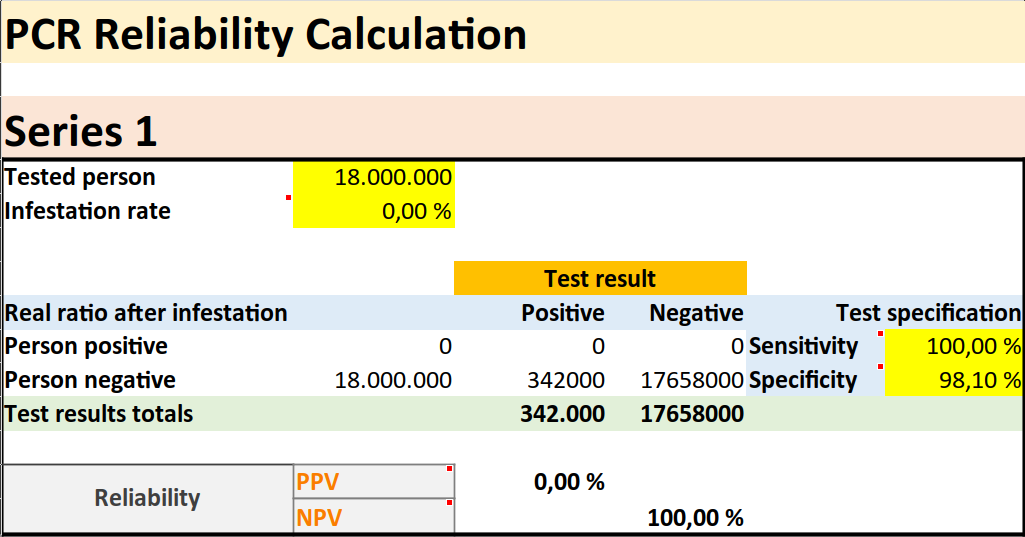
A small or even non-existent infection, together with many tests carried out, inevitably leads to a high number of false positives and can create the impression of an epidemic or pandemic. We find in the PCR tests, therefore, the main cause of the corona saga. This is a highly error-prone test that produces many false positive results even in the complete absence of a new disease. It is by no means a coincidence that the crisis really took off after the WHO Director-General advised all the world's governments at a press conference on March 16, 2020 (25):
"We have a simple message for all countries: test, test, test."
This should become the mantra of the following months. Testing, testing, testing was the credo in this pandemic, on the grounds that this was the only way to determine how strongly the virus would spread. On the contrary! It is rather the case that the spread of the allegedly infected one to one is accompanied by the spread of the tests.
Dr. David Rasnick a US American biochemist said:
"You have to have a whooping amount of any organism to cause symptoms. Huge amounts of it. You don't start with testing: you start with listening to the lungs. I'm skeptical that a PCR test is ever true. It's a great scientific research tool. It's a horrible tool for clinical medicine. 30% of your infected cells, have been killed before you show symptoms. By the time you show symptoms [...] the dead cells are generating the symptoms."
Asked if one should have oneself tested, Dr. David Rasnick replied:
"Don't do it. I say, when people ask me. No healthy person should be tested. It means nothing but it can destroy your life, make you absolutely miserable."
TRUE SITUATION

In the graph above we see the number of tests performed, the number of positive results, and the number of deaths with positive tests for Germany. Currently, the mass media and the government are fanning fears of a so-called second wave. For this the rising so-called infection numbers, thus the positive or false positive results of the tests are shown. As we can see, since the beginning of august the number of tests carried out daily has increased considerably. Therefore, after all we have looked at, it is not at all surprising that the alleged infection numbers are increasing. More tests more false positive results.
Please read on → here