EVENING SERVICE
Evening song 🎶
For the oval dinner, the monastic brethren of the rapists sing the second part from the Valaam monastery with Father Archimandrite Methodius, who wants a bon appetit and a luxurious evening!
https://t.me/Tribulelouis/5487
There is a house in New Orleans
The Rising Sun is called
Ruin he became a lot of poor guys.
I know, God, I'm one of them here!
My mom was a dressmaker,
Made new blue jeans for me
And my father was an avid dashing gambler.
Throughout New Orleans
And now only one player needs
Luggage and his suitcase
And only then he was pleased -
When he was drunk in the insole
O mother, tell your children,
Not to do what I'm in it.
and so on and so forth...

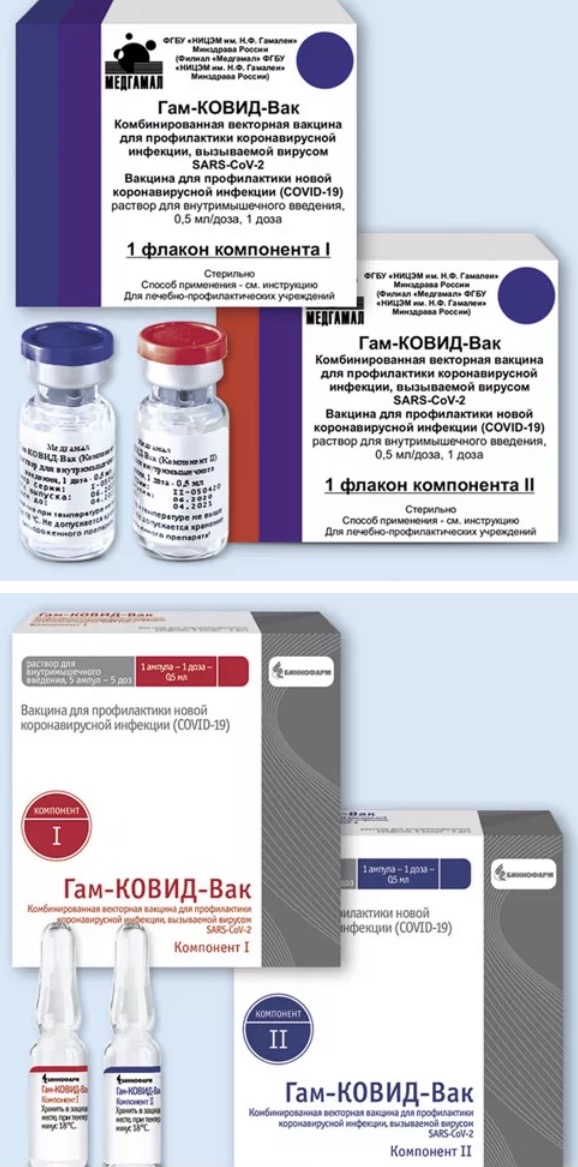
CLASSIFIED INFORMATION AND DOCUMENTS FOR THE DRUG GAM-COVID-VAC (SPUTNIK V)
Please note that all documents say "drug", not "vaccine".
Also pay attention to the patent, it says, the drug for animals (mammals). That is, those who are offered to inject are considered as a biological species of "mammals", and not as a reasonable person.
📜 Instructions for use Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
💊 Composition of Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
🚩Use of Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
📅 Storage Conditions Gam-COVID-Vac Combined Vector Vaccine for the Prevention of CORONAVIRUS Infection caused by SARS-CoV-2
⏳ Shelf Life Gam-COVID-Vac Combined Vector Vaccine for the Prevention of Coronavirus Infection Caused by SARS-CoV-2
Information consent









Patent Pdf
https://t.me/Tribulelouis/5492
Instructions for use
RESPONSIBILITY OF MEDICAL WORKERS
FOR NOT WARNING PEOPLE THAT THE SO-CALLED VACCINATION
FROM COVID-19 IS A TRIAL OF UNEXPLORED MEDICINAL
DRUG (THIRD STAGE: HUMAN TRIAL)
The introduction of the drug Gam-COVID-Vac-2020 (Sputnik V) into the human body is not vaccination, but is a study of this medical drug on humans, conducted by the Federal State Budgetary Educational Center named after N. F. Gamaleya (the third stage of trials), as evidenced by the "Information sheet of the study participant with an informed consent form" dated 01.09.2020 on 16 sheets, which must be provided to the trial participant for review and signature.
In case of non-notification of a person before the so-called vaccination and failure to notify him of this document for signature, the medical worker is responsible for the following articles of the Criminal Code
Of the Russian Federation:
1. Criminal Code of the Russian Federation Article 115. Intentional infliction of light harm to health
Art. 1. Intentional infliction of light harm to health, which caused a short-term health disorder or a slight permanent loss of general working capacity, shall be punished by a fine in the amount of up to forty thousand rubles or in the amount of wages or other income of the convicted person for a period of up to three months, or compulsory labor for up to four hundred and eighty hours, or corrective labor for up to one year, or arrest for up to four months.
2. Criminal Code of the Russian Federation Article 112. Intentional infliction of moderate severity of harm to health
Art. 1. Intentional infliction of moderate severity of harm to health, not dangerous to human life and not entailing the consequences specified in Article 111 of this Code, but causing a long-term health disorder or a significant permanent loss of general working capacity by less than one third, shall be punished by restriction of freedom for up to three years, or forced labor for up to three years, or arrest for up to six months. , or imprisonment for up to three years.
Article 2. The same act committed: a) in respect of two or more persons; c) in respect of a minor or other person, obviously for the guilty person who is in a helpless state; d) by a group of persons, a group of persons by prior agreement or an organized group, - shall be punished by imprisonment for up to five years.
3. Criminal Code of the Russian Federation Article 111. Intentional infliction of grievous harm to health
Art. 1. Intentional infliction of grave harm to health, dangerous to human life, or entailed loss of vision, speech, hearing or any organ or loss of its functions by the body, termination of pregnancy, mental disorder, disease of drug addiction or substance abuse, or expressed in indelible disfigurement of a person, or caused a significant permanent loss of general working capacity by at least one third or knowingly for the perpetrator a complete loss of professional working capacity. Ti, - punishable by imprisonment for up to eight years.
Art. 2. The same acts committed: b) in relation to a minor or other person, obviously for the guilty person who is in a helpless state; d) for hire, - punishable by imprisonment for up to ten years with restriction of liberty for up to two years or without it.
Art. 3. Acts provided for in the first or second parts of this Article, if they are committed: a) by a group of persons, a group of persons by prior agreement or an organized group; b) in respect of two or more persons, - shall be punished by imprisonment for up to twelve years with restriction of liberty for up to two years or without it.
Art. 4. Acts, provided by parts one, two or three of this Article, entailed by negligence death of the victim - shall be punished by imprisonment for up to fifteen years.
Description of the drug Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by the SARS-CoV-2 virus
Based on the officially approved instructions for use of the drug and prepared for the electronic edition of the Vidal handbook 2020, update date: 2021.05.17
Release form, packaging and composition Clinical and pharmacological. group Pharmaco-therapeutic group Pharmacological action Indications of the drug Dosage regimen Side effect Contraindications to use Special instructions Overdose Drug interaction Conditions of dispensing from pharmacies Storage conditions Shelf life
Marketing Authorization Holder:
FSBI "NITsEM named after N.F. Gamaleya" of the Ministry of Health of Russia (Russia)
Produced by:
GENERIUM, JSC (Russia) or PHARMSTANDART-UfaVITA, JSC (Russia) or FSBI "NITsEM named after N.F. Gamaleya" of the Ministry of Health of Russia (branch of "MEDGAMAL" FSBI "NITsEM named after N.F. Gamaleya" of the Ministry of Health of Russia) (Russia ) or BIOCAD, CJSC (Russia) or R-PHARM, JSC (Russia) or BINNOFARM, JSC (Russia) or LEKKO, CJSC (Russia)
ATX code: J07B (Vaccines for the prevention of viral infections)
Active substance: vaccine for preventing of new coronavirus infection caused by the SARS-CoV-2 virus
Group Grouping name
Dosage forms
Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
Solution for intramuscular injection, component I - 0.5 ml / dose (frozen preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 0.5 ml / dose (frozen preparation): amp. 5 pieces.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 0.5 ml / dose (frozen preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 0.5 ml / dose (frozen preparation): amp. 5 pieces.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 0.5 ml / dose (frozen preparation): vial. 1, 2, 5 or 10 pcs.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 0.5 ml / dose (frozen preparation): vial. 1, 2, 5 or 10 pcs.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 0.5 ml / 1 dose (liquid preparation): amp. 5 pieces.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 0.5 ml / 1 dose (liquid preparation): amp. 5 pieces.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 3.0 ml / 5 doses (frozen preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 3.0 ml / 5 doses (frozen preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 3.0 ml / 5 doses (frozen): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 3.0 ml / 5 doses (frozen): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component I - 3.0 ml / 5 doses (liquid preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Solution for intramuscular injection, component II - 3.0 ml / 5 doses (liquid preparation): vial. 1 PC.
reg. No: LP-006395 dated 08/11/2020 - Current
Re-registration date: 18.02.21
Release form, packaging and composition of the drug Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
Solution for i / m administration (component I and component II).
Frozen preparation
Component I. The frozen solution is a dense, solidified, whitish mass. After thawing: a homogeneous colorless or slightly opalescent solution with a yellowish sheen.
1 dose (0.5 ml)
recombinant adenoviral particles of serotype 26 containing the gene for protein S of the SARS-CoV-2 virus (1.0 ± 0.5) × 1011 particles
Excipients: tris (hydroxymethyl) aminomethane - 1.21 mg, sodium chloride - 2.19 mg, sucrose - 25.0 mg, polysorbate 80 - 250.0 μg, magnesium chloride hexahydrate - 102.0 μg, EDTA disodium salt dihydrate - 19.0 μg, ethanol 95% - 2.5 μl , water d / i - up to 0.5 ml.
0.5 ml (1 dose of component I) - bottles (1) - cardboard packs.
0.5 ml (1 dose of component I) - bottles (1, 2, 5, 10) - cardboard packs.
3.0 ml (5 doses of component I) - vials (1) - cardboard packs with a holder.
3.0 ml (5 doses of component I) - 6R vials (1) - cardboard packs.
3.0 ml (5 doses of component I) - 2R vials (1) - contoured cell packs (1) - cardboard packs.
0.5 ml (1 dose of component I) - ampoules of colorless glass with a colored dot (5) - contoured cell packs (1) - cardboard packs.
0.5 ml (1 dose of component I) - colorless glass ampoules (5) - contoured cell packs (1) - cardboard packs.
Component II. The frozen solution is a dense, hardened, whitish mass. After thawing: a homogeneous colorless or slightly opalescent solution with a yellowish sheen.
1 dose (0.5 ml)
recombinant adenoviral particles of serotype 5 containing the protein S gene of the SARS-CoV-2 virus (1.0 ± 0.5) × 1011 particles
Excipients: tris (hydroxymethyl) aminomethane - 1.21 mg, sodium chloride - 2.19 mg, sucrose - 25.0 mg, polysorbate 80 - 250.0 μg, magnesium chloride hexahydrate - 102.0 μg, EDTA disodium salt dihydrate - 19.0 μg, ethanol 95% - 2.5 μl , water d / i - up to 0.5 ml.
0.5 ml (1 dose of component II) - bottles (1) - cardboard packs.
0.5 ml (1 dose of component II) - vials (1, 2, 5, 10) - cardboard packs.
3.0 ml (5 doses of component II) - vials (1) - cardboard packs with a holder.
3.0 ml (5 doses of component II) - 6R vials (1) - cardboard packs.
3.0 ml (5 doses of component II) - 2R vials (1) - contoured cell packs (1) - cardboard packs.
0.5 ml (1 dose of component II) - ampoules of colorless glass with a colored dot (5) - contoured cell packages (1) - cardboard packs.
0.5 ml (1 dose of component II) - colorless glass ampoules (5) - contoured cell packs (1) - cardboard packs.
Liquid preparation
Component I. Homogeneous colorless or slightly opalescent solution with a yellowish sheen.
1 dose (0.5 ml)
recombinant adenoviral particles of serotype 26 containing the gene for protein S of the SARS-CoV-2 virus (1.0 ± 0.5) × 1011 particles
Excipients: tris (hydroxymethyl) aminomethane - 1.21 mg, sodium chloride - 2.19 mg, sucrose - 25.0 mg, polysorbate 80 - 250.0 μg, magnesium chloride hexahydrate - 102.0 μg, EDTA disodium salt dihydrate - 19.0 μg, ethanol 95% - 2.5 μl , water d / i - up to 0.5 ml.
3.0 ml (5 doses of component I) - vials (1) - cardboard packs with a holder.
3.0 ml (5 doses of component I) - vials (1) - contoured cell packs (1) - cardboard packs.
0.5 ml (1 dose of component I) - colorless glass ampoules (5) - contoured cell packs (1) - cardboard packs.
Component II. Homogeneous colorless or slightly opalescent solution with a yellowish tinge.
1 dose (0.5 ml)
recombinant adenoviral particles of serotype 5 containing the protein S gene of the SARS-CoV-2 virus (1.0 ± 0.5) × 1011 particles
Excipients: tris (hydroxymethyl) aminomethane - 1.21 mg, sodium chloride - 2.19 mg, sucrose - 25.0 mg, polysorbate 80 - 250.0 μg, magnesium chloride hexahydrate - 102.0 μg, EDTA disodium salt dihydrate - 19.0 μg, ethanol 95% - 2.5 μl , water d / i - up to 0.5 ml.
3.0 ml (5 doses of component II) - vials (1) - cardboard packs with a holder.
3.0 ml (5 doses of component II) - vials (1) - contoured cell packs (1) - cardboard packs.
0.5 ml (1 dose of component II) - colorless glass ampoules (5) - contoured cell packs (1) - cardboard packs.
Clinical and pharmacological group: Vaccine for the prevention of coronavirus infection caused by the SARS-CoV-2 virus
Pharmaco-therapeutic group: MIBP vaccine
pharmachologic effect
▼ This medicinal product is registered under the registration procedure for medicinal products intended for use in conditions of threat of occurrence, occurrence and elimination of emergency situations. The instruction was prepared based on the limited amount of clinical data on the use of the drug and will be supplemented as new data becomes available. The use of the drug is possible only in the conditions of medical organizations that have the right to carry out vaccination of the population in the prescribed manner.
Characteristic. The vaccine is obtained by biotechnology, which does not use the SARS-CoV-2 virus, which is pathogenic for humans. The drug consists of two components: component I and component II. Component I includes a recombinant adenoviral vector based on human adenovirus serotype 26 carrying the gene for protein S - SARS-CoV-2 virus; component II includes a vector based on human adenovirus serotype 5 carrying the protein S gene of SARS-CoV-2 virus ...
The vaccine induces the formation of humoral and cellular immunity against coronavirus infection caused by the SARS-CoV-2 virus.
Immunological efficacy
The immunological properties and safety of the vaccine were studied in various clinical studies in adult volunteers of both sexes over the age of 18 years.
An interim immunogenicity analysis showed that the vaccine elicits an immune response in volunteers. When studying the humoral immune response, the sera of the volunteers were analyzed for the presence of antibodies specific to the receptor-binding domain of the S glycoprotein of the SARS-CoV-2 virus on the 42nd day from the start of vaccination: in the group of vaccinated, the geometric mean of the antibody titer was 8996, the seroconversion level was 98.25%. When comparing the level of RBD-specific antibodies between age strata, a statistically significant difference was shown for the 18-30 year old group relative to other age groups: the geometric mean of the antibody titer was 18102-22067, the seroconversion level was 100%. Antibody levels did not differ significantly between men and women. On the 42nd day from the start of vaccination, the mean geometric titer of neutralizing antibodies in the immunized volunteers was 44.47, the seroconversion level was 95.83%. There was no statistically significant difference in volunteers of different sex and age.
Immunization with Gam-COVID-Vac forms a strained antigen-specific cellular anti-infectious immunity in almost all examined volunteers (the formation of antigen-specific cells of both populations of T-lymphocytes: T-helper (CD4 +) and T-cytotoxic (CD8 +) and a significant increase in the secretion of IFNγ ).
The protective antibody titer is currently unknown. The duration of the protection is unknown.
Clinical studies to study the epidemiological effectiveness are ongoing. According to the data of the interim analysis, the efficiency is more than 91% /
Indications of the drug Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
Prevention of novel coronavirus infection (COVID-19) in adults over 18 years of age.
Dosage regimen
The vaccine is intended for intramuscular administration only. Intravenous administration of the drug is strictly prohibited. The vaccine is injected into the deltoid muscle (the upper third of the outer shoulder). If it is impossible to inject into the deltoid muscle, the drug is injected into the vastus lateral muscle.
Vaccination is carried out in two stages: first with component I at a dose of 0.5 ml, then after 3 weeks - with component II at a dose of 0.5 ml.
After the vaccine is administered, the patient should be monitored by a healthcare professional for 30 minutes.
Application of the vaccine produced by NF Gamaleya NITsEM of the Ministry of Health of Russia (Medgamal branch of NF Gamaleya NITsEM of the Ministry of Health of Russia) (vials) and Binnopharm AO (ampoules) and R -Pharm "(vials)
Before vaccination, a vial or ampoule with component I or II is taken out of the freezer and kept at room temperature until completely thawed. Remaining ice inclusions are not allowed! Wipe the bottle or ampoule from the outside with an alcohol napkin to remove moisture. Mix the contents gently by shaking. Shaking the bottle or ampoule is not allowed!
Remove the protective plastic cover from the bottle and treat the rubber stopper with an alcohol napkin.
The ampoule is opened at the colored dot.
Using a disposable syringe with a needle, a 0.5 ml dose is withdrawn for administration to the patient.
Storage of the thawed preparation is not allowed!
Re-freezing is not allowed!
Application of the vaccine produced by JSC "GENERIUM" (bottles), JSC "Pharmstandard-UfaVITA" (bottles, ampoules) and JSC "LEKKO" (bottles)
Frozen preparation
Before vaccination, a vial or ampoule with component I or II is taken out of the freezer and kept at room temperature until completely thawed. Remaining ice inclusions are not allowed! Wipe the bottle or ampoule from the outside with an alcohol napkin to remove moisture. Mix the contents gently by shaking. Shaking the bottle or ampoule is not allowed!
Remove the protective plastic cover from the bottle and treat the rubber stopper with an alcohol napkin. Open the ampoule along the ring or break point.
Using a disposable syringe with a needle, a 0.5 ml dose is withdrawn for administration to the patient.
If subsequent injections are postponed for any reason, it is allowed to store the opened vial or ampoule - no more than 2 hours at room temperature. Repeated freezing of a bottle or ampoule with a solution is not allowed!
Liquid preparation
Attention! Do not use a vial or ampoule with a drug with visible defects in the closure system and / or glass. Freezing a bottle or ampoule with a solution is not allowed!
Preparation of solution for injection. The vial or ampoule is taken out of the refrigerator and kept at room temperature, it is allowed to slightly heat the drug, for example, by holding it in your hands. Do not heat the drug above 37 ° C.
Remove the protective plastic cover from the bottle and treat the rubber stopper with an alcohol napkin or open the ampoule along the ring or break point.
Using a disposable syringe with a needle, a 0.5 ml dose is withdrawn for administration to the patient.
If subsequent injections are postponed for any reason, it is allowed to store the opened vial or ampoule for no more than 2 hours at room temperature.
Application of the vaccine produced by CJSC "BIOCAD" (vials)
Before vaccination, the vial with component I or II is taken out of the freezer and kept at room temperature until completely thawed. Remaining ice inclusions are not allowed! Wipe the outside of the bottle with an alcohol napkin to remove moisture. Mix the contents gently by shaking. Shaking the bottle is not allowed!
Remove the protective plastic cover from the bottle and treat the rubber stopper with an alcohol napkin.
Using a disposable syringe with a needle, a 0.5 ml dose is withdrawn for administration to the patient.
If subsequent injections are postponed for any reason, it is allowed to store an opened 3 ml vial for no more than 2 hours at room temperature.
Storage of the thawed drug in 0.5 ml vials is not allowed!
Re-freezing is not allowed!
Attention! A preparation (liquid and / or frozen) with defects in the closure system and / or broken labeling of the vial or ampoule, with a change in the physical properties of the solution (turbidity, color), improper storage and / or with an expired shelf life is unsuitable for use.
▼ Information for healthcare professionals performing vaccination with a medicinal product: this medicinal product is registered under a special registration procedure, and therefore it is necessary to notify the Federal Service for Supervision of Healthcare about each fact of using the medicinal product by entering information into the appropriate section of the information system of the Unified State Health Information System.
Side effect
Adverse events characteristic of the use of the vaccine, identified in clinical trials, as well as studies of other vaccines based on a similar technological platform, are mainly mild or moderate, can develop in the first or second days after vaccination and are resolved within 3 subsequent days. More often than others, short-term general (short-term flu-like syndrome characterized by chills, fever, arthralgia, myalgia, asthenia, general malaise, headache) and local (soreness at the injection site, hyperemia, swelling) reactions may develop. It is recommended to prescribe non-steroidal anti-inflammatory drugs (NSAIDs) with an increase in temperature after vaccination and antihistamines with the severity of a local reaction.
Less commonly noted: nausea, dyspepsia, decreased appetite, sometimes - an increase in regional lymph nodes. Some patients may develop allergic reactions, a short-term increase in the level of hepatic transaminases, creatinine and creatine phosphokinase in the blood serum.
In the framework of the conducted clinical studies of the safety, tolerability and immunogenicity of Gam-COVID-Vac after vaccination, the following adverse events (AEs) were recorded.
General disorders and reactions at the injection site: very often and often - hyperthermia, pain, edema, itching at the vaccination site, asthenia, pain, malaise, pyrexia, increased skin temperature at the vaccination site, decreased appetite.
Disturbances from the respiratory system, chest and mediastinal organs: often - pain in the oropharynx, nasal congestion, sore throat, rhinorrhea.
Nervous system disorders: often - headache; rarely - dizziness, fainting.
Gastrointestinal disorders: often - nausea, vomiting, dyspepsia.
Laboratory and instrumental data: multidirectional deviations of immunological status indicators - an increase in the number of T-lymphocytes, an increase in the percentage of lymphocytes, a decrease in the number of natural killer cells, an increase in the number of CD4-lymphocytes, a decrease in the number of CD4-lymphocytes, an increase in the number of B-lymphocytes, a decrease in the number of B - lymphocytes, an increase in the number of natural killer cells, an increase in the number of CD8 lymphocytes, an increase in the level of immunoglobulin E (IgE) in the blood, an increase in the CD4 / CD8 ratio, a decrease in the CD4 / CD8 ratio, an increase in the level of immunoglobulin A (IgA) in the blood, a decrease in the percentage the content of CD8 lymphocytes. Deviations in the general blood test - an increase in the percentage of lymphocytes, a decrease in the hematocrit, an increase in the number of lymphocytes, an increase in the erythrocyte sedimentation rate, an increase in the number of leukocytes, an increase in the number of monocytes, an increase in the number of platelets, a decrease in the number of neutrophils, a decrease in the number of platelets. Deviations in the general analysis of urine - erythrocytes in the urine.
Most AEs resulted in recovery without consequences. Laboratory deviations had no clinical significance (the vaccinated did not need additional diagnostic procedures and therapy).
Contraindications for use
hypersensitivity to any component of a vaccine or vaccine containing similar components;
history of severe allergic reactions;
acute infectious and non-infectious diseases, exacerbation of chronic diseases - vaccination is carried out 2-4 weeks after recovery or remission. With mild ARVI, acute infectious diseases of the gastrointestinal tract, vaccination is carried out after the temperature has returned to normal;
pregnancy;
period of breastfeeding;
age up to 18 years (due to the lack of data on efficacy and safety).
Contraindications for the introduction of component II:
severe post-vaccination complications (anaphylactic shock, severe generalized allergic reactions, convulsive syndrome, temperature above 40 ° C, etc.) for the administration of component I of the vaccine.
Carefully
Use the vaccine with caution in chronic liver and kidney diseases, endocrine diseases (severe thyroid dysfunction and
diabetes mellitus in the stage of decompensation), severe diseases of the hematopoietic system, epilepsy and other diseases of the central nervous system, acute coronary syndrome and acute cerebrovascular accident, myocarditis, endocarditis, pericarditis.
Due to the lack of information, vaccination can pose a risk for the following patient groups:
with autoimmune diseases (stimulation of the immune system can lead to an exacerbation of the disease, especially patients with autoimmune pathology, which tends to develop severe and life-threatening conditions, should be treated with caution);
with malignant neoplasms.
The decision to vaccinate should be based on an assessment of the balance of benefits and risks in each specific situation.
Application during pregnancy and lactation
The drug is contraindicated during pregnancy and during breastfeeding, because its effectiveness and safety during this period have not been studied.
Application for violations of liver function
Use with caution in patients with chronic liver disease.
Application for impaired renal function
Use with caution in patients with chronic kidney disease.
Application in children
Contraindication: age up to 18 years (due to the lack of data on efficacy and safety.
special instructions
Patients receiving immunosuppressive therapy and immunocompromised patients may not develop a sufficient immune response. Therefore, taking drugs that suppress the function of the immune system is contraindicated for at least 1 month before and after vaccination due to the risk of reduced immunogenicity.
Influence on the ability to drive vehicles and mechanisms
Studies to study the effect of the vaccine on the ability to drive vehicles and potentially dangerous mechanisms have not been conducted.
Overdose
No cases of overdose have been reported.
Considering that the dispensing of the drug is allowed only for medical institutions, and the vaccination itself is carried out only by qualified medical personnel, the risk of overdose is extremely low.
However, it can be assumed that in case of an accidental overdose, the development of the above toxic and toxic-allergic reactions to a more severe degree is possible.
There are no specific antidotes to the drug.
Therapeutic measures in this case will include symptomatic therapy in accordance with the indications (antipyretic / NSAIDs and desensitizing agents, parenteral corticosteroids for severe toxic-allergic syndrome). The regimen of prescribing drugs should be selected according to the recommendations for use and dosages of this drug.
Drug interactions
Has not been studied.
Storage conditions of the drug Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
Frozen preparation
Storage conditions: store in a dark place at a temperature not exceeding minus 18 ° С. Re-freezing is not allowed.
Keep out of the reach of children.
Shelf life of the drug Gam-COVID-Vac Combined vector vaccine for the prevention of coronavirus infection caused by SARS-CoV-2
Expiration date: component I - 6 months; component II - 6 months. Do not use after the expiration date.
Transportation conditions: transportation of the drug at a temperature not higher than minus 18 ° С.
Liquid preparation
Storage conditions: store in a dark place at a temperature of 2 ° to 8 ° C. Do not freeze.
Keep out of the reach of children.
Expiration date: component I - 2 months; component II - 2 months. Do not use after the expiration date.
Transportation conditions: transportation of the drug at a temperature of 2 ° to 8 ° C. Do not freeze.
Terms of sale
For medical institutions.
https://www.vidal.ru/drugs/gam-covid-vac
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)
The examination is carried out by the company "Crocus Medical B.V." LLC - a branch of the Dutch Crocus Medical BV.
Full legal name: BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)
Head: MANAGING DIRECTOR BUTYLIN ALEKSEY ALEKSANDROVICH
INN / KPP: 9909177837
Authorized capital: 0.001 thd.
Number of founders: 1
CONTACT INFORMATION:
Legal address: 105064, MOSCOW, KAZAKOVA STREET, 6, BUILDING 1, OFFICE 202
Phone: 8 (495) 917-45-61
E-mail:
website:
COMPANY DETAILS:
INN: 9909177837
Ppc:
OKPO: 78510877
OGRN: 0
OKFS: 23 - Property of foreign legal entities
OKSU: 4210011 - Business entities and partnerships with the participation of foreign legal entities and (or) individuals, as well as stateless persons
OKOPF: 30002 - Branches of legal entities
OCTMO: 45375000000
OKATO: 45286555 - Basmanny, Central, Moscow
Activities:
Main (according to OKVED code): 85.11 - Activities of medical institutions
Find similar companies - in the same industry and region (with the same OKVED and OKATO)
Additional activities under OKVED:
65.23 Financial intermediation n.e.c.
70.31 Activities of real estate agencies
73.10 Research and development in the natural and technical sciences
74.14 Business and management advice
74.84 Provision of other services
85.12 Medical practice
85.14 Other health activities
PUBLIC PROCUREMENT UNDER 44-FZ NOT FOUND
PUBLIC PROCUREMENT UNDER 223-FZ NOT FOUND
ARBITRAGE: DISABLE THE AD BLOCKER
CERTIFICATES OF CONFORMITY: DISABLE THE AD BLOCKER
ENFORCEMENT PROCEEDINGS: DISABLE THE AD BLOCKER
BRIEF INFORMATION:
Organization 'BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)' registered at the address 105064, Moscow, KAZAKOVA STREET, 6, BUILDING 1, OFFICE 202. The company was issued a TIN 9909177837. The main activity is the activity of medical institutions. The company is headed by MANAGING DIRECTOR Aleksey Aleksey A.
Kazakhstan
Almaty
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)
Activities of professional affiliates
Bin
130241009775
· VAT
60001-0085115
Full name
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)
Name in Kazakh
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "CROCUS MEDICAL B.V." (NETHERLANDS)
Name in English
KROKUS MEDIKAL B.V.
status
░░░░ ░░░░ ░░░░ ░░░
Legal form
Branch of a private limited liability company
address
Kazakhstan, Almaty, Almaty
Bin
130241009775
Company size
Small Businesses
Registration date
08.02.2013 · 8 years 5 months
Leaders
Butylin Aleksey Aleksandrovich
Form of ownership
private property
Founders
░░░ ░░░░░░ ░░░░░ ░░░░░░
Number of employees
< = 5 people
Tax authority
6007 · UGD in Almaly district
Date of last re-registration
08.05.2021
Branches
BRANCH OF A PRIVATE LIMITED LIABILITY COMPANY VITOL SERVICES B.V. (NETHERLANDS) IN THE REPUBLIC OF KAZAKHSTAN · BIN 130941020583
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "ENI ISATAY B.V." · BIN 150541021605
BRANCH OF THE COMPANY "SAIGAK KAZAKHSTAN B.V." · BIN 100941010888
WAGENBORG KAZAKHSTAN B.V. KAZAKHSTAN BRANCH · BIN 980941014456
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "AGRO PAK B.V." ("AGRO PAK B.V.") · BIN 040241008311
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "KMG KASHAGAN B.V." IN THE REPUBLIC OF KAZAKHSTAN · BIN 060441007176
BRANCH OF THE LIMITED LIABILITY COMPANY "AGIP KARACHAGANAK B.V." · BIN 980741000518
AKTOBE BRANCH OF ALTIES PETROLEUM B.V. · BIN 010241001329
KAZAKHSTAN BRANCH OF THE COMPANY - "COOPER INDUSTRIES GLOBAL B.V." · BIN 120541020389
KAZAKHSTAN BRANCH OF NELSON PETROLEUM BUZACHI B.V. · BIN 990341000901
ATYRAU BRANCH OF ALTIES PETROLEUM INTERNATIONAL B.V. · BIN 990941001199
BRANCH OF PRIVATE LIMITED LIABILITY COMPANY "BEKAZ B.V." (BEKAZ B.V.) IN THE REPUBLIC OF KAZAKHSTAN · BIN 080141000796
ZHAMBYL BRANCH OF PRIVATE COMPANY "CANADIAN BACON LTD" · BIN 200641018982
https://statsnet.co/companies/kz/59682456
Araz Iskender oglu Agalarov (Azerbaijani: Araz Iskender oglu Agalarov; Araz İskəndər oğlu Ağalarov; born November 8, 1955, Baku) is a Russian and Azerbaijani businessman, president and owner of the Crocus Group.[1] In 2020, he took 55th place in the Forbes rating "200 richest businessmen of Russia" with a fortune of $ 1.7 billion[2].
The company "Crocus International" businessman Araz Agalarov without a competition received two state contracts for the placement of 1500 beds for COVID-19 patients in the exhibition complex "Crocus Expo" in the Moscow region. This is reported by "Present Time" and the investigative project Scanner Project.
Both contracts are concluded with the Krasnogorsk City Clinical Hospital No. 1. Their total amount is 1.035 billion rubles. Thus, one hospital place in crocus Expo cost the hospital 690 thousand rubles. The rent of the premises of the exhibition center for the hospital is paid until the end of June 2020. The contract itself is valid until December 2020.
The basis for the deal was the decree of the Governor of the Moscow region Andrei Vorobyov on the introduction of a high-alert regime in the region. This made it possible to conclude contracts for the refurbishment of Crocus Expo premises without a tender and public hearings.
As noted by "Present Time" and scanner Project, the contracts were concluded shortly after Araz Agalarov on April 21 complained to RBC about the financial difficulties of his holding Crocus Group. He said that the holding spends monthly on salaries (Crocus Group employs 15 thousand people) almost a billion rubles. According to Agalarov, the group's resources will allow paying salaries for March, but there will be no money for April.
In total, the government of the Moscow region spent 3.6 billion rubles on the repair and preparation of hospitals in the region to receive infected with COVID-19 in April: 34 contracts with developers were posted on the public procurement website. But the contract amount with Crocus Expo is nearly a third of all urgent investment in medical infrastructure to fight the coronavirus in April 2020. The money 💰 just stole. Everything was empty.
On April 27, the Governor of the Moscow region, Andrei Vorobyov, announced that additional hospitals for coronavirus patients will be opened in the region in the Patriot Park and the Crocus City Hall concert hall. Later, the first vice-president of Crocus Group and the son of Araz Agalarov Emin Agalarov said that the hospital was deployed not in Crocus City Hall, but in Crocus Expo.
The temporary hospital on the territory of Crocus Expo was a series of boxes-"wards" from mobile partitions. There are also boxes for bathrooms and for connecting medical equipment. In fact, this is not a hospital, but a large ……(((
Doctors near Moscow Agalarov hospital told about the shortage of medicines and specialists
20:57 23.05.2020 under the heading Society MAX57 2417 23 1

Doctors near Moscow Agalarov hospital told about the shortage of medicines and specialists
Doctors working in a temporary hospital for coronavirus patients, deployed in the Crocus Expo exhibition complex near Moscow by businessman Araz Agalarov, spoke about the lack of medicines, equipment, personal protective equipment (PPE) and qualified personnel. Although the hospital has been working for two weeks, the first employment contracts with nurses began to be concluded only now, and the salary rate is reduced.
The temporary hospital opened on 12 May. Contracts worth more than 1 billion rubles for the deployment of 1500 beds were concluded without competition with Agalarov's company Crocus International shortly after the businessman complained of financial difficulties. The basis for the conclusion of contracts was the decree of the Governor of the Moscow region Andrei Vorobyov on the introduction of a high-alert regime in the region.
"Present time" and the investigative project Scanner Project found out that after the opening of the first temporary hospital with Agalarov signed contracts for another 876 million rubles, that is, the total amount of contracts approached 2 billion rubles. Under the new contracts, it is planned to deploy another 1100 beds, rent the Expo Hotel for patients and doctors and supply hot meals to patients.
The temporary hospital is managed by the City Clinical Hospital N1 of Krasnogorsk, its doctors and nurses work in Crocus Expo, about whom colleagues speak very well. In addition to professionals, specialists who have undergone retraining in the conditions of the epidemic are involved in the work. Students of non-core universities and colleges work as orderlies: lawyers, engineers, plumbers, sellers.
According to doctors, the temporary hospital was not an observatory for the lungs of patients, as stated initially: people from the risk group are also brought there. There are serious patients, including those connected to ventilators. One of the doctors said that there is a staff who, "in principle, does not know how to do anything - out of ten nurses, one can get into a vein."
After the first week of the hospital's work, personal protective equipment began to run out: "those same uneducated orderlies" soaked removable filters for protective masks in chlorine, because of which they lost their functionality, the doctor said. Employees also told reporters about the shortage of some medicines, later some of the drugs and necessary devices were admitted to the hospital.
The converted exhibition hall is arranged so that the compartments for patients are open from above, while ventilation works, which, according to doctors, nullifies the division of the hospital into "red" and clean zones. Officially, there are no COVID-19 cases there. "Not a single patient has yet been confirmed with coronavirus in Crocus: all have community-acquired bilateral pneumonia polysegmental," said one of the interlocutors of journalists.
One of the patients praised the conditions in the hospital, but noted the lack of medicines and qualified personnel. According to him, the doctor, whose treatment helped the patient, was suspended from work, for the fact that "demanded too many drugs, and Crocus could not buy them in a timely manner." Also, patients say that the hospital does not have CT machines, drugs for blood thinning, and treatment is reduced to prescribing medicines for malaria.

Donald Trump and Aras Agalarov before the final of the contest "Miss Universe - 2013" in Crocus City Hall.

Sberbank is one of the sponsors of the contest.



The Nazis agitate people to join the boycott of Jewish shops, 1933.




Pity the guys, their stomachs hurt with laughter 😆
Here is a good hot topic of the day and year!
https://telegra.ph/UK-government-accused-of-murdering-pensioners-07-26


President Lincoln with Major General McClernand and Alan Pinkerton, 1862.
ꪑꪚ. ଓꪚꪋꪱꪀઽ