See This Report about LEPU Medical
Getting My TABLE-EU agrees list of COVID-19 rapid tests - Thomson To Work
Gold-labelled SARS-Co, V-2 N protein monoclonal antibodies incapacitated on the test area, with corresponding antibodies in the quality control location. Swabbed specimens from the nasopharynx or oropharyngeal, result in 15 minutes. Item Functions Non-invasive Simple to utilize Convenient, no gadgets required Rapid, get a result in 15 minutes Steady, with high accuracy Inexpensive, cost-efficiency, Related News, On 26th Jan 2021, report from PEI of an official test that The Federal Institute for Drugs and Medical Devices (Bundesinstitut fr Arzneimittel und Medizinprodukte, Bf, Ar, M) licensed to carry out, declared that Lepu Medical SARS-Co, V-2 Antigen Rapid Test Kit has actually satisfied all the minimum requirements for antigen tests conducted by PEI in assessment with the Robert Koch-Institut (RKI).
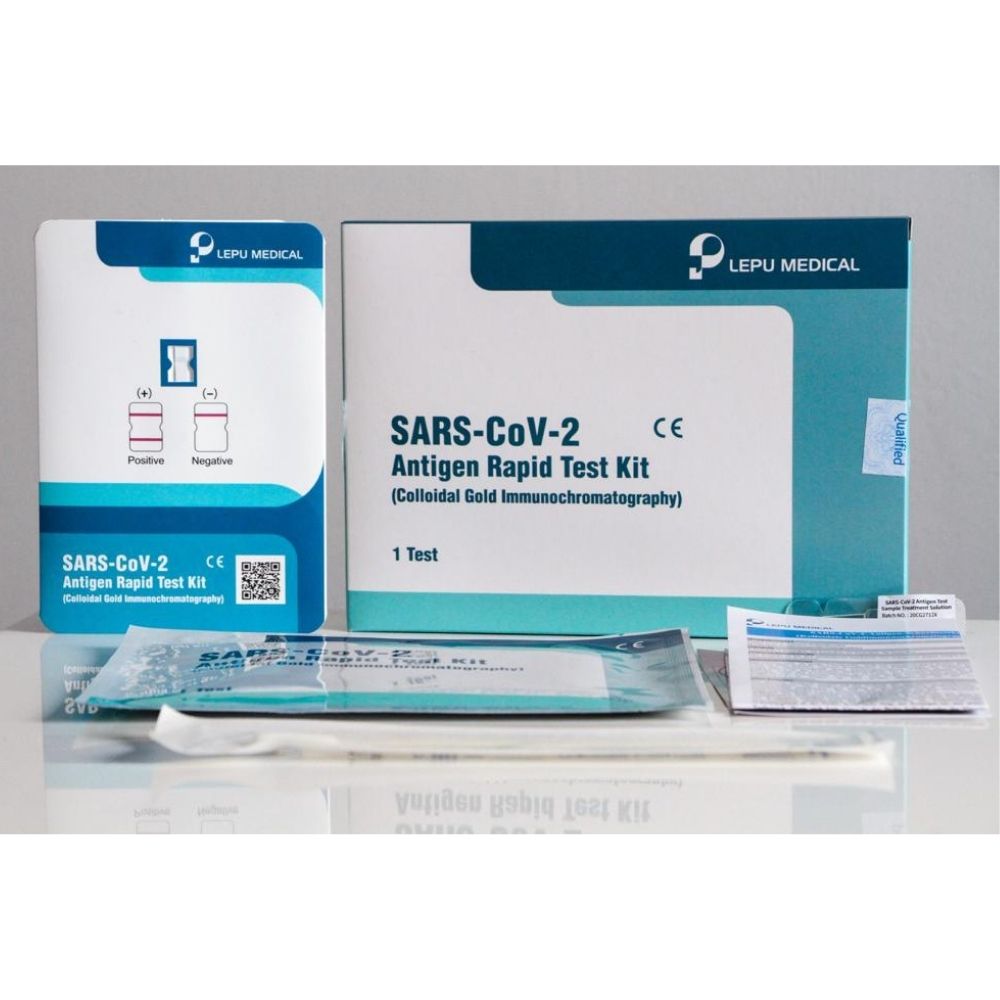 LEPU Medical COVID Rapid Test Professional Use – 25 pcs - Experts Express Shop
LEPU Medical COVID Rapid Test Professional Use – 25 pcs - Experts Express ShopThe test kit makes the coronavirus detection procedures quick and simple.
On Might 28, 2021, the FDA alerted customers not to use the Lepu Medical Innovation SARS-Co, V-2 Antigen Fast Test Kit and the Leccurate SARS-Co, V-2 Antibody Fast Test Kit (Colloidal Gold Immunochromatography) due to a "high threat of incorrect results when using these tests." These tests have been distributed to drug stores to be sold for at-home screening by consumers along with marketed directly to consumers in the U.S.
What Does COVID-19 SELF TEST KITS Mean?(For info about FDA authorized COVID tests, including at-home tests, see Consumer, Lab's post about Discovering the very best COVID Tests). This Piece Covers It Well provided a recall of these tests on April 26, 2021. The recall includes all lots of the tests, consisting of at least at least 8,419,545 antibody tests and 205,175 antigen tests distributed from roughly March 20, 2020 to today.

 Lepu Medical POC Testing SARS-CoV-2 Antigen Rapid Test Kit Manufacturer/Company, Immunochromatograph
Lepu Medical POC Testing SARS-CoV-2 Antigen Rapid Test Kit Manufacturer/Company, ImmunochromatographThe FDA advises people who have used these tests and have issues about their test results to seek advice from their health care company.
CH Tralee is devoted to maintaining the accuracy of the active ingredient lists on this website. However, because active ingredients go through change, we can not ensure that these lists are total, current and/or error-free. For a precise listing of components in each product, please refer to your item packaging.
An Unbiased View of SARS-CoV-2 - MaxanimDetailn popis produktu Rychl test je in vitro diagnostick test pro kvalitativn detekci antigen koronaviruptomnch v nosohltanu, a to za. Identifikace jespecifickch pro antigen novho koronaviru. Kdy se pouv je akutn respiran infekn onemocnn. Cover jsou obecn nchyln. V souasnosti jsou hlavnm zdrojem infekce pacienti infikovan novm koronavirem; asymptomatit infikovan lid mohou bt tak infeknm zdrojem.