What makes supercritical carbon dioxide an exceptional SCFE solvent?
Thar ProcessA supercritical fluid is a fluid that is highly-compressed fluid that merges the properties of gases along with liquids. Supercritical fluids have an assortment of supporting technologies used in more than a few manufacturing applications.
They figure the foundation of unsoiled technology as a substitute solvent for extracting natural goods, chemicals, and additional substances.
Applying temperature along with pressure higher than the critical point of a substance pushes those substances into the supercritical stage. The properties of this liquid can be adjusted by going additional into the supercritical area. This is attained by mounting the temperature or else pressure.
For instance, mounting the temperature above 31°C as well as pressure higher than 73 bar for Carbon dioxide creates a supercritical stage, neither liquid nor even gas but a mixture of together properties. High dispersal is like a gas other than with the salvation (capacity to liquefy substances) of a liquid. Further treatment of these circumstances can be used to generate a diversity of only one of its kind properties used to take out a number of usual products.
Supercritical carbon dioxide (CO2) is a kind of fluid state of CO2 where it is heated as well as held at or higher than its critical temperature plus pressure. In this supercritical stage, CO2 displays assets and behaviors among that of a liquid plus a gas. In exacting, supercritical CO2 possesses liquid resembling thickness with gas-like diffusivity, exterior tension, and stickiness.
When CO2 go beyond temperatures of 87.9°F (31.1°C) and is subjected to pressures higher than 1071 psi (7.39 MPa), it comes in the supercritical phase. This phase of CO2 is usually used as a solvent in chemical removal processes due to its elevated solubility, small toxicity, and least net result on the environment.
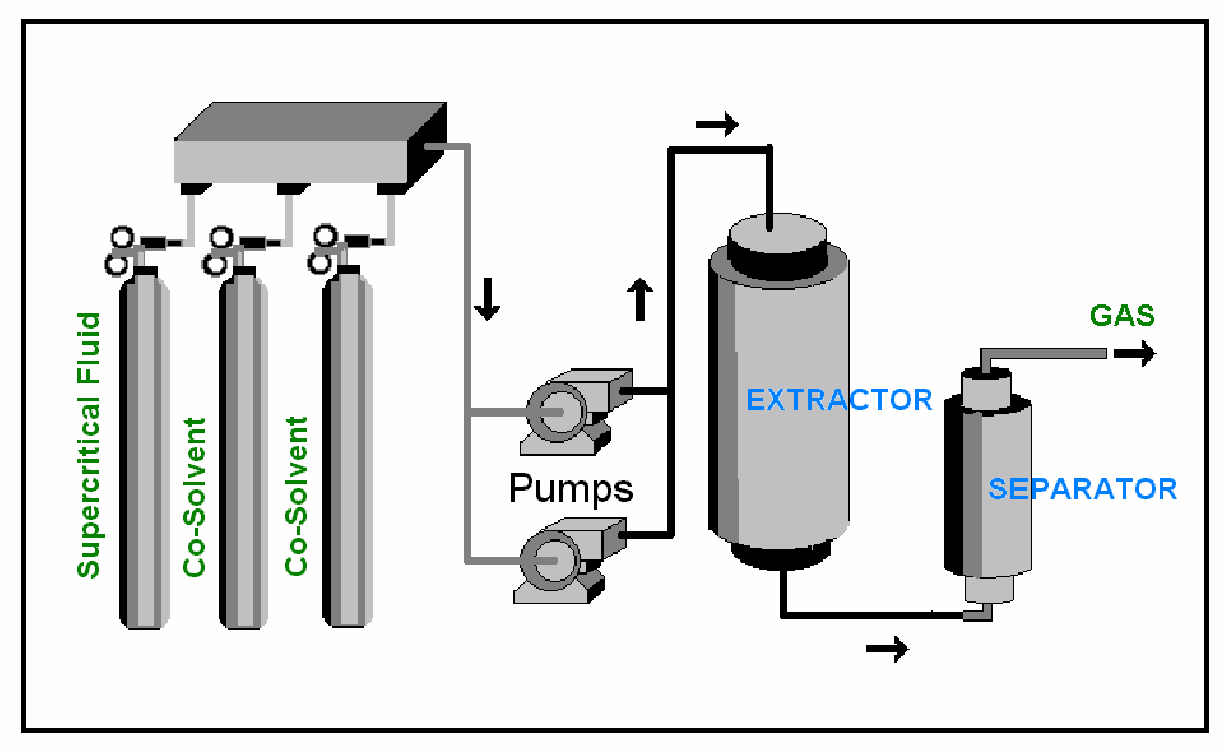
Supercritical carbon dioxide (sCO2) is the main broadly used liquid for the Supercritical Extraction (SCFE) procedure across the world. Not only is sCO2 able of extracting the necessary component in their perfect form, the major reason for the rolling status of SCFE, it also makes the procedure more convenient, inexpensive, and protected.
The properties of CO2 are well recognized and can be oppressed for numerous helpful technical purposes and manufacturing processes. For instance, the thickness of supercritical CO2 can be altered noticeably by little changes in the weight or temperature about the critical point. CO2 thickness is very low and the exterior tension of supercritical CO2 is absent. Diffusivity is elevated, which in a mixture with low thickness induces important changes in strong phases.
As well, supercritical Chromatography CO2 pressure the belongings of machinery with which it is varied For instance, supercritical CO2 can melt many nonpolar compounds far away from its steam pressure. Also, an important quantity of supercritical CO2 can liquefy into condensed phases severely reducing the outside tension and thickness of the condensed stage making processing of sticky equipment easier.
Carbon dioxide with water are a good number widely engaged supercritical extraction makes such a helpful supercritical fluid as it:
- Contain a critical temperature of 31.10C, which is approximately the ambient temperature.
Civilizing its compatibility along with temperature receptive compounds;
- Has a more convenient critical pressure of about 73.9 bar;
- Is non-flammable as well as non-toxic;
- Has a customizable thickness to improve its solvent control;
- Is accessible in sample quantities within the clean form; and
- Has a moderately low cost.
- Even though CO2 is a greenhouse gas (GHG), the SCFE procedure using CO2 becomes ecological if the gas is captured from the ambiance and used.
Solvent Extraction utilizes organic solvents to liquefy the component from the red material. Later, it divides the organic solvent from the dissolved component. The problem is, natural solvents do not totally disassociate from the component, leaving after residues.
That is, the component loses its natural clarity. What is more, it may expand a sure degree of toxicity. But the concerns about using usual solvents for extraction go away from toxicity for the person. Several solvents are ozone-reducing substances and their uses harmfully impact the environment.
To know more visit Thar Process.