Understanding the Electromagnetic Spectrum
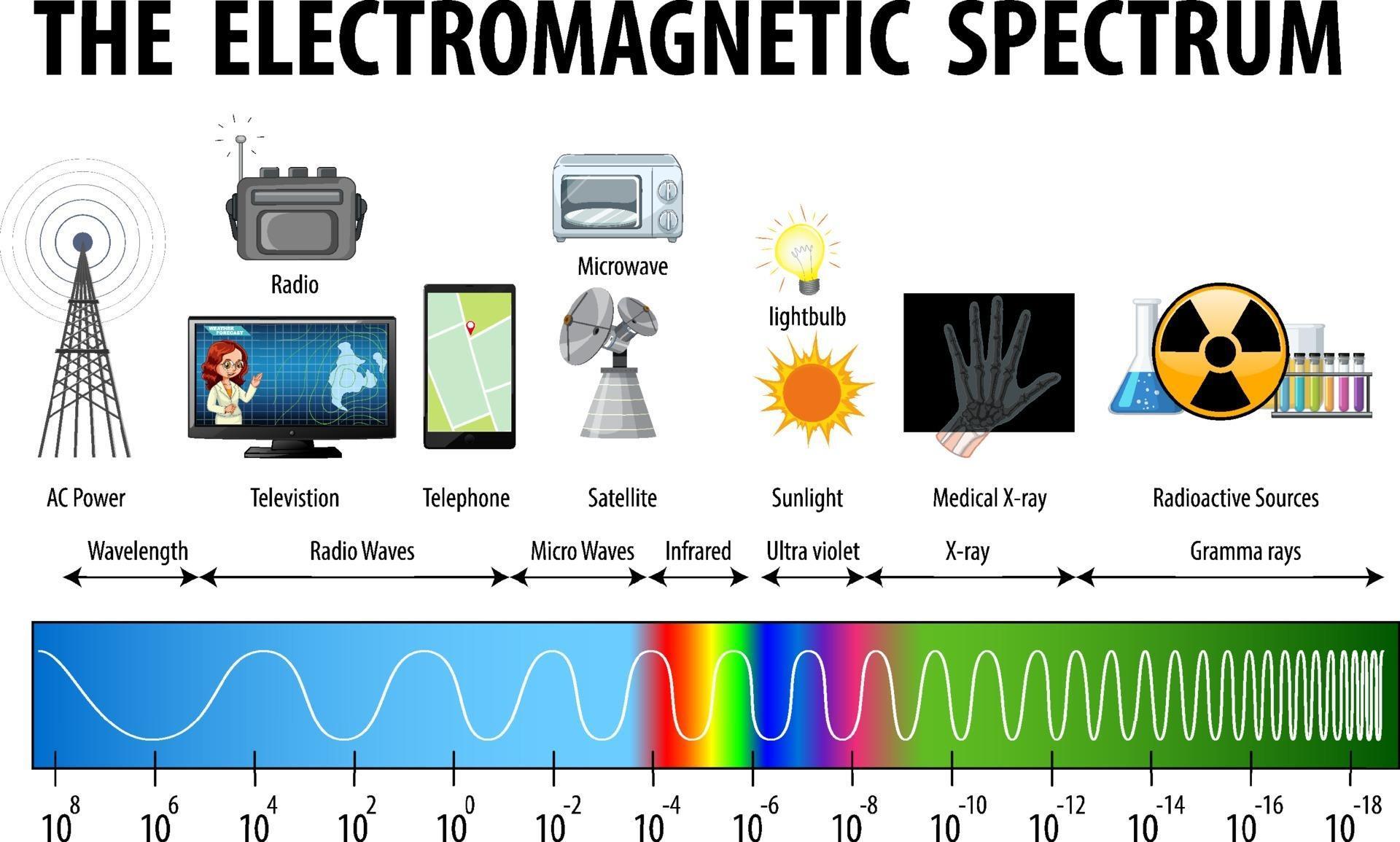
The electromagnetic spectrum is a description of the spectrum of electromagnetic waves that range from the visible light to gamma radiation. This is an important component of science and understanding the electromagnetic spectrum is crucial. In this article I will go over some of the most important aspects of this range as well as how they work.
Infrared
Infrared refers to the spectrum that radiates electromagnetic radiation which extends past the red end of the visible light spectrum. Infrared spectrum is utilized to assess the thermal properties of objects. It can also be used for night-vision equipment.
Generally, infrared is classified into near infrared and infrared. Near infrared is the wavelength that contains the shortest frequencies. These wavelengths are in the range of one to five microns. There are also intermediate and long infrared bands. Each has the unique wavelengths.
The most well-known use for infrared is found in military night vision goggles. These goggles convert infrared into visible wavelengths for night-time viewing. Infrared light, however, is also used for wireless and wired communications.
There is no evidence of a link between infrared radiation and skin cancer. However, there is a link between infrared and skin cancer. International Commission on Non-Ionizing Radiation Protection (ICNIRP) has issued guidance on the exposure limits to invisible visible and infrared radiation.
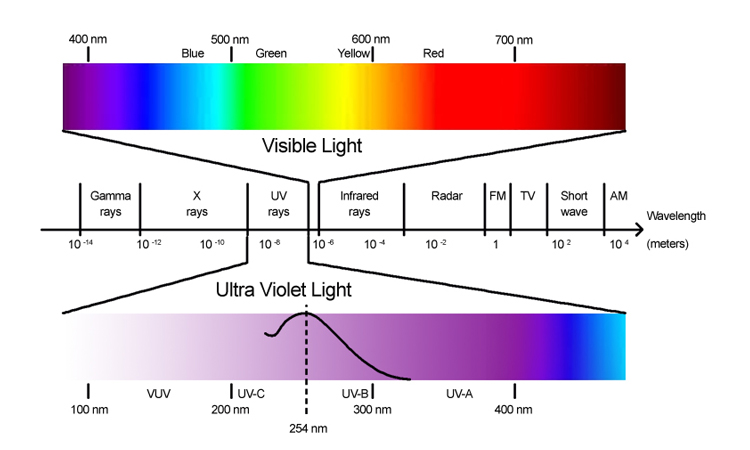
Visible light
Visible light is part in the spectrum known as electromagnetic. The Sun is the main lighting source. Other sources of visible light are the moon as well as the stars. It is important to know that we can't see the ultraviolet and infrared wavelengths. But, we can see the red and blue light. These colours are blended together to create what is known as white light.
There are numerous other obscure components to electromagnetic spectrum such as infrared and radio waves. Some of these have been utilized for radio, television and mobile communications. However, the most effective way to utilize these is to create the right type of filter. By doing so, we can reduce the negative consequences of these elements to our bodies. Additionally, we can build an environment in which it is safe to study these components, even without the use of our eyes.
While the shortest and longest wavelengths of visible light may be the most visible but the most efficient and pleasing to the eye include the shorterwave infrared (SWIR) and microwave frequencies.
UV
Ultraviolet (UV) radiation is part of the electromagnetic spectrum. It can be utilized for various purposes. However, it is also dangerous. UVB and UVC radiation are not good for the human eye, and can lead to skin cancer.
The energy generated by this type of source can be absorbed by atoms and initiate chemical reactions. The absorbing molecule can then produce visible light, or even fluoresce.
The spectrum of ultraviolet light is divided into three categories: the extreme, near, and the far. Typical ultraviolet sources include lasers, arc lamps and light-emitting diodes.
Although UV rays have wavelengths that are shorter, UV radiations are less than those of X-rays they possess more energy. This is useful for breaking chemical bonds. The waves are also referred to by the name of nonionizing radiation.
In biochemistry, the UV spectrum is commonly used to measure the absorption rate of a particular substance. There are numerous types of substances with significant absorption bands of light in the UV.
Ultraviolet light is a member of the electromagnetic spectrum which is produced through the sun. Its spectrum spans between 10 and 4100 nanometres and the frequencies range from 800 THz to 30 PHz. But, the majority of people can't see it.
X-rays
X-rays are electromagnetic radiation with high energy. Unlike gamma rays and ultraviolet light, X-rays are less than visible light and they can penetrate relatively thin objects. They are employed in a myriad types of applications in medicine, including imaging bones and tissues. Several types of X-rays exist.
Hard X-rays are produced when an incoming electron collides against an atom. The result is a void within the electron shell of the atom. Another electron could fill in the gap. Or, the electron that is incoming could release an atom. When this happens, part of the energy of the photon is transferred to the scattering electron.
An X-ray is not to be mistaken for the X-band, which is a low-energy part of the electromagnetic spectrum. Although both bands are separated by a few centimeters in size, they don't have the same characteristics.
Because X-rays are penetrating, they can be used in a myriad of ways. For instance, X-rays are utilized in security screening to find cracks in luggage. In addition, they are employed in radiotherapy to treat cancer patients. They are also employed to identify the structural elements of materials such as cement.
Gamma rays
Gamma rays are extremely high-energy types in electromagnetic radiation. In fact, all extremely high energy photons are rays. These photons are created by nuclear decay and high-energy physical experiments. They are among the most energetic photons that are found in the electromagnetic spectrum.
Due to their powerful energy, gamma rays are capable of reaching far into the material. In fact, it is feasible for a gamma ray to penetrate several inches of lead.
Many high-energy physics experiments create gamma rays. For example, a particle beam from a relativist centered by the magnetic field of hypernovas can be observed at 10-billion light years.
Certain gamma rays are released by the nucleus in some radionuclides following their passage through the process of radioactive decay. Other sources of gamma rays include atomic transitions or annihilation as well as sub-atomic particle interactions.
Gamma rays in the majority in astronomy come from different mechanisms. Gamma rays emitted by supernovae and nuclear fallouts are some of the most energetic forms of electromagnetic radiation. This makes them an excellent source for exploring the universe.
Some gamma rays may cause damage to cells within the body. However, gamma rays are not as ionizing as beta and alpha rays, which means they are less likely to cause cancer. Nevertheless, electromagnetic spectrum with wavelength and frequency can alter the DNA structure and cause burns. Even the smallest amounts of gamma radiations could cause Ionization within the body.