Sex Sex Hormone Download And Sex

🛑 👉🏻👉🏻👉🏻 INFORMATION AVAILABLE CLICK HERE👈🏻👈🏻👈🏻
This site uses cookies. By continuing to browse this site you are agreeing to our use of cookies.
Originally published9 Mar 2017https://doi.org/10.1161/ATVBAHA.116.307301Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37:746–756
You are viewing the most recent version of this article. Previous versions:
January 1, 2017: Previous Version 1
This review summarizes recent evidence concerning hormonal and sex chromosome effects in obesity, atherosclerosis, aneurysms, ischemia/reperfusion injury, and hypertension. Cardiovascular diseases occur and progress differently in the 2 sexes, because biological factors differing between the sexes have sex-specific protective and harmful effects. By comparing the 2 sexes directly, and breaking down sex into its component parts, one can discover sex-biasing protective mechanisms that might be targeted in the clinic. Gonadal hormones, especially estrogens and androgens, have long been found to account for some sex differences in cardiovascular diseases, and molecular mechanisms mediating these effects have recently been elucidated. More recently, the inherent sexual inequalities in effects of sex chromosome genes have also been implicated as contributors in animal models of cardiovascular diseases, especially a deleterious effect of the second X chromosome found in females but not in males. Hormonal and sex chromosome mechanisms interact in the sex-specific control of certain diseases, sometimes by opposing the action of the other.
Cardiovascular diseases (CVDs) manifest differently in men and women.1,2 The overall lifetime risk of CVD is similar in the 2 sexes, but men develop CVD earlier than women (Figure 1).3,4 At 55 years of age, the lifetime risk of a first incident coronary heart disease is higher in men than in women, but the risk of first incident cerebrovascular disease or heart failure is higher in women than in men.3 These sex differences suggest that biomedical principles, learned from the study of males, may not apply equally to females. CVDs should be studied in both sexes. However, separate study of both sexes is not enough. Rather, the 2 sexes must be directly compared, with the purpose of finding factors that cause sex differences and, therefore, prevent or alleviate disease in one sex more than the other. The present review summarizes limited evidence about the causes of sex differences in CVDs in humans and recent investigations of rodent models in which causal factors can be isolated and studied in a more controlled manner. Although there is no expectation that mice and other model organisms are equivalent to humans, studies of diverse organisms, which are similar to and different from humans, is the main strategy for formulating basic biological concepts that guide our understanding of human physiology. In the present case, studies of mice have recently uncovered new ideas that have yet to be applied to humans. Investigation of animals offers 2 distinct advantages. The first is the ability to independently manipulate diverse sex factors (those that cause sex differences in physiology and disease, including hormones and numerous specific sex chromosome genes) to judge their separate effects, as well as their interactions. The second advantage is the ability to discover downstream molecular mechanisms controlled by sex factors, which might be targets for therapy. The ideas presented here include an emerging field of research based on study of animals. The new ideas will require validation and application to humans, to achieve better understanding of human physiology and disease.
Although sex differences in CVDs are caused in part by environmental or social differences between men and women (eg, occupational hazards, habits, social stresses),5–7 we focus here on biological factors that are important and tractable for study in animal models.
Where do sex differences start? At the moment of conception, XX and XY zygotes differ only in their sex chromosomes. Thus, all sex differences arise from the inherent sexual inequality of these 2 chromosomes.8 We list here 4 classes (2 Y-linked and 2 X-linked) of sex chromosome mechanisms that could conceivably cause sex differences in phenotype.
The most important sex-differentiating effect is caused by the Y-linked gene Sry, which acts within gonadal primordia to cause differentiation of testes in males.9 Genes that are present in both sexes cause differentiation of ovaries unless Sry is present, so Sry expression is the root cause of sex differences in the type of gonads. The differentiation of testes rather than ovaries establishes a lifelong difference in the levels of gonadal hormones, especially testosterone, estradiol, and progesterone, which act directly on cardiovascular and other tissues to make them function differently in the 2 sexes. Some effects of testosterone (or its metabolite 17β-estradiol) are permanent (organizational effects), for example, the hormone-induced male pattern of differentiation of the genitals or brain. Others are reversible (activational effects) and may last only as long as the hormone is present.10
Y chromosome genes act outside of the gonads to have male-specific effects. For example, Sry is expressed in the brain and other tissues, especially in catecholaminergic cells and neurons, where its effects may include effects on hypertension.11–13 Studies of XY mice with different versions of the Y chromosome show remarkable differences in the severity of autoimmune disease.14 Effects of the Y chromosome must be specific to males, but the implied sex difference could be compensated for by other factors in females. These effects have to date been infrequently studied because historically it has been difficult to target the Y chromosome or manipulate it without also changing gonadal hormone levels. Moreover, this chromosome encodes relatively few genes, and except for Sry, none has been specifically implicated yet in sex differences in physiology.
Most X chromosome genes do not show large sex differences in their level of expression, because one X chromosome is transcriptionally silenced in XX adult somatic cells. Thus, most X genes are expressed about equally from the single active X chromosome in both XX and XY cells. Accordingly, concepts of sexual differentiation have long ignored X chromosome genes as potential inherent causes of sex differences in phenotype. However, some genes escape inactivation and are expressed from each X chromosome so that they have higher levels in XX than in XY cells.15–18 Among these are a set of X chromosome genes known to be highly dosage sensitive,19 implying that one versus 2 doses of the genes are expected to cause phenotypic differences. In mice and humans, the X escapees include 2 histone demethylases, Kdm5c and Kdm6a, which are likely to have widespread effects on autosomal gene expression. Other X escapees also have fundamental effects on cell function and include the translation initiation factor Eif2s3x and RNA helicase Ddx3x, which is involved in transcriptional regulation, pre-mRNA splicing, mRNA export, cellular signaling, and viral replication. The X escapees are candidates for causing sex differences in CVD, but none has been specifically implicated to date.
XY cells receive a maternal imprint on X genes, but XX cells receive imprints from both parents. The inherent imbalance in parental imprinting is a potential cause of sex differences in expression of specific X genes that affect CVD phenotypes. To date, this difference in imprinting has not been specifically implicated as the cause of any sex difference in phenotype, possibly because this class of genes is not widely studied, and proving their role in sexual differentiation is experimentally complex.
In addition to the effects listed here, there may be nongenic effects (ie, not requiring gene expression) of the X or Y chromosomes that alter the epigenetic status of the autosomes. These mechanisms are speculative and are discussed elsewhere.20
In recent years, a major goal has been to distinguish sex differences caused by gonadal hormones versus sex chromosome complement (XX versus XY). Hormonal effects are most often detected by manipulating hormone levels, synthesis, or action to find which hormones account for sex differences.21 Detecting nongonadal effects of sex chromosome complement involves manipulating the number of X and Y chromosomes, holding gonadal hormones as constant as possible. The most frequently used model is the four core genotypes (FCG) mouse model.22 In this model, Sry is a transgene on chromosome 3 and is not present on the Y chromosome, so that XX and XY mice can each have either testes (with Sry, XXM or XYM) or ovaries (without Sry, XXF or XYF; Figure 2). This model can discriminate between sex differences determined by gonadal type (XXM and XYM differ from XXF and XYF) versus those determined by the effects of sex chromosomes (XXF and XXM differ from XYF and XYM).
Once a sex difference is found to be caused by a sex chromosome effect, the next step is to figure out if the effect is caused by the X or Y chromosome, using the XY* mouse model (Figure 2). The Y* chromosome has a variant pseudoautosomal region that recombines abnormally with the X chromosome, producing mice with unusual complements of sex chromosomes. For example, some progeny have a fusion of an X and Y chromosome, whereas others lack most of one X chromosome.23,24 The progeny are the near equivalents of XO, XX, XY, and XXY. This model allows detecting an effect of 1versus 2 X chromosomes, by comparing XO versus XX (gonadal females) or XY versus XXY (gonadal males). One can also detect an effect of the Y chromosome by comparing XO versus XY and XX versus XXY.25 Localizing the effect to the X or Y chromosome then leads to studies of candidate X or Y genes that might cause the sex chromosome effect.
Previous reviews discuss methods and interpretation of these models, including methods to avoid hormonal confounds of sex chromosome complement.22,26 Below, we review studies of gonadal hormone and sex chromosome influences on obesity, atherosclerosis, aneurysms, cardiac ischemia/reperfusion, and hypertension.
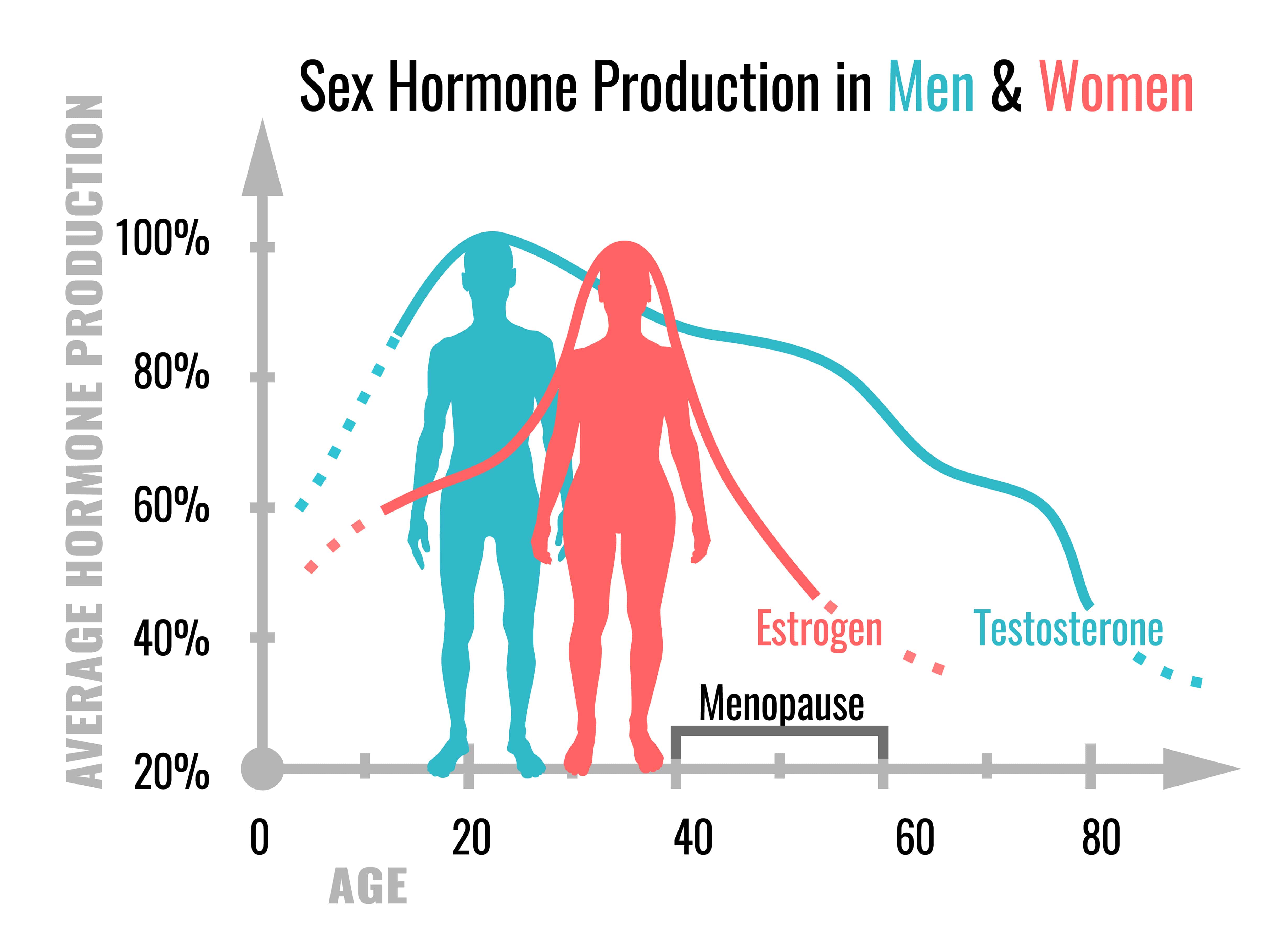
Many CVDs are associated with obesity. Men and women differ in the development of obesity, and the manifestation of obesity-related conditions such as hyperlipidemia, insulin resistance, and type 2 diabetes mellitus. Men generally have greater body weight than women, but the proportion of body weight as fat is greater in women.27–29 In the mouse, males also have greater body weight, but the degree of body fat is dependent on diet and strain. In some strains, a high-fat diet leads to similar adiposity, whereas in others one sex has greater adiposity than the other.30,31 Multiple mechanisms contribute to sex differences in fat storage, including ability to expand different anatomic depots (eg, greater subcutaneous fat storage in women) or ability to mobilize fat stores (eg, greater capacity for adipose tissue lipolysis in men).27–29 Additional sex differences include greater insulin sensitivity and higher adiponectin and leptin levels in women and female mice, and lower dietary fatty acid oxidation and greater circulating triglyceride levels in men.30,32 Some of the metabolic differences between men and women are reduced after menopause, possibly caused by the decline in ovarian hormones.33 However, hormonal differences cannot fully account for all differences between men and women in lipid metabolism.34
Genome-wide genetic studies in humans have identified genetic loci that influence body mass index and waist-hip-ratio specifically in one sex or to a different extent in the 2 sexes. These include loci on the autosomes and on the X chromosome.35,36 In the mouse, numerous genetic loci with differential effects in males and females have been described, including several loci on chromosome X.30,37 The mechanisms underlying sex-specific effects of genetic polymorphisms on obesity merit further study.
The use of experimental models such as the FCG and XY* mice (Figure 2) have informed us about genetic and hormonal mechanisms underlying sex differences in obesity.17 In gonad-intact FCG mice, male mice (with testes, either XX or XY) have greater body weight than female mice (with ovaries, XX and XY; Figure 3A). In addition, XX mice weigh more than XY mice of the same gonadal sex. After gonadectomy of adult mice, male–female sex differences are reduced, indicating that acute effects of gonadal hormones contribute to male–female differences in body weight. Weeks or months after gonadectomy, however, the body weight of XX mice increases more than that of XY mice, and XX mice eventually have nearly twice as much body fat.17 These results suggest that the presence of 2 X chromosomes, and/or the absence of the Y chromosome, leads to enhanced body fat. The XY* model resolves this question in favor of the X chromosome dose. Mice with 2 X chromosomes (XX or XXY) have greater body weight than mice with one X chromosome (XY or XO; Figure 3B).17 XX mice after gonadectomy also have accelerated weight gain on a high-fat diet, with increased food intake, relative to XY, during the light phase of the diurnal cycle, but with no differences in energy expenditure or locomotor activity.17,38 XX mice also develop more profound fatty liver, greater evidence of insulin resistance, and higher circulating cholesterol levels than XY mice when stressed with fat- or cholesterol-enriched diets.17,38 Thus, the number of X chromosomes has a substantial effect on obesity and related morbidities.
Localizing the sex chromosome effect to the X chromosome opens the door to identifying novel X-linked molecular determinants of sex differences in obesity. A leading hypothesis suggests a contribution from X escapee genes, which are expressed at higher levels in XX compared with XY tissues.17 Testing this hypothesis further will involve assessing the effects on obesity when expression levels of individual X escapee genes are modulated in a controlled manner.
Among all CVDs, coronary artery disease is the leading cause of death. Over 2 centuries ago, clinicians began to report sex differences in the prevalence of angina pectoris.39 In general, women have a delay in the development of atherosclerosis compared with men, with both sexes increasing in incidence with age.40 A recent meta-analysis suggests that increased total serum cholesterol is a significant risk factor for coronary artery disease in both sexes, with a small, but significantly stronger effect in men compared with women.41 Women have higher high-density lipoprotein (HDL) cholesterol levels and lower bile acid and cholesterol synthesis than men throughout adult life.42 Although sex differences in lipoprotein levels persist after menopause, endogenous sex hormone levels influence lipoprotein levels. For example, postmenopausal women have higher low-density lipoprotein (LDL) cholesterol than premenopausal women or men of the same age.43–45 Furthermore, ovariectomy increases LDL cholesterol levels.46 Among individuals, the level of plasma estrone correlates with HDL cholesterol levels and correlates inversely with total cholesterol.47,48
Testosterone may influence atherosclerosis in both sexes. In postmenopausal women of the Atherosclerosis Risk in Communities study, who were not taking hormone therapy, the level of sex hormone–binding globulin, which binds ≈60% of circulating testosterone, correlated with a more favorable lipid profile (lower total and LDL cholesterol and higher HDL cholesterol). Thus, relative androgen excess during menopause, rather than loss of estrogen, may adversely affect lipid status of women.49 Conversely, testosterone has been suggested to protect males from atherosclerosis.50–53 Males with higher circulating testosterone concentrations had higher HDL cholesterol levels.54,55 In studies tracking serum lipids and lipoproteins from childhood to adulthood, levels of HDL cholesterol dropped in boys but were not changed in girls after puberty.56,57 These results suggest effects of endogenous testosterone during development to regulate HDL cholesterol. Moreover, the decline in testosterone levels with age58 is associated with age-dependent increases in atherosclerosis in males,59–61 although other age-related factors could be causal. Serum sex hormone–binding globulin concentrations correlate inversely with atherosclerosis in men.62,63 These studies offer tantalizing suggestions that endogenous androgens may have opposite or divergent effects in males and females, but further research is needed.
Even more controversial than these effects of endogenous sex hormones are the effects of exogenous sex hormone therapy on coronary artery disease. In women with established coronary artery disease, administration of combined conjugated equine estrogen plus medroxyprogesterone acetate therapy increases the risk of heart disease events at 1 year.64 In the large Women’s Health Initiative, continuous treatment with conjugated equine estrogen plus medroxyprogesterone acetate resulted in significantly increased risk of coronary artery events and stroke.65,66 Finally, in women with hysterectomies administered unopposed estrogen (specifically, oral conjugated equine estrogen, 0.625 mg/d) for the prevention of chronic postmenopausal conditions, there were no benefits on coronary heart disease, with an increased risk for venous thromboembolism and stroke.67 It is important to note that the number of risk factors present in the study population and other differences in study design (eg, use of statins) may have influenced the cardiovascular outcomes of these studies. Recent results from the ELITE (Early versus Late Intervention Trial with Estradiol) highlights the potential importance of timing of initiation of 17β-estradiol therapy relative to menopause.68 Oral 17β-estradiol therapy was associated with less progression of subclinical atherosclerosis when therapy was initiated within 6 years after menopause but not when therapy was initiated ≥10 years after menopause. These differential effects of estradiol therapy might be related to changes in estrogen receptor expression in pivotal target tissues such as the vasculature. Current guidelines continue to caution against use of postmenopausal hormone therapy to prevent cardiovascular events in women. However, given the conflicting conclusions reached from these various studies, an understanding of cardiovascular risk in women requires further research on hormone treatment in terms of type and delivery method of estrogen and progesterone, as well as the timing and duration of treatment.
Inbred mouse strains are valuable for varying sex and other variables when genetic background is held constant. Mice and humans differ in lipid profile, with mice carrying the majority of blood cholesterol in HDL particles, whereas humans have much greater l
2021 Sex Karya
Anus After Sex
Bestiality Sex Breasts Hentai
Https Pornogo Sex Porno Tv
Sex Klip Turma 2021 Porno
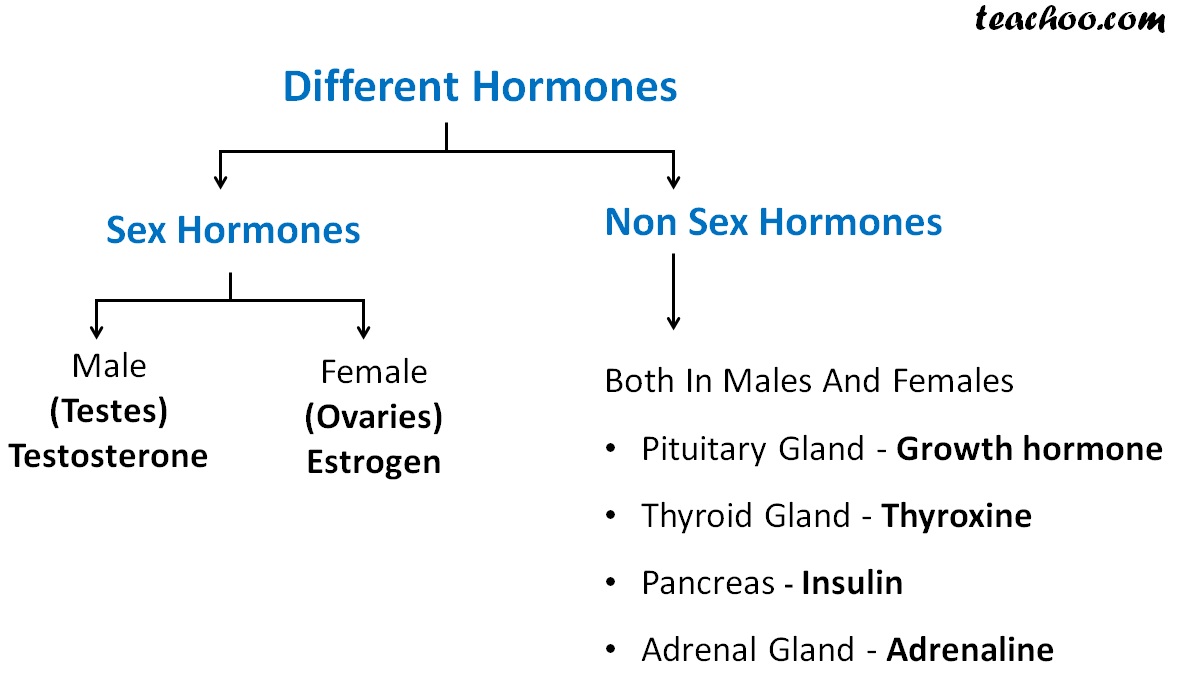
Sex Hormones - Types & Functions of Sex Hormones
Sex Hormones and Sex Chromosomes Cause Sex Differen…
Sex Hormones | IntechOpen
SexLab Hormones (May 2021) - Sex Effects - LoversLab
SexLab Hormones (May 2021) - Downloads - SexLab Framework ...
(PDF) Hormones, Sex, and Gender - researchgate.net
(PDF) Sex in the brain: Hormones and sex differences
(PDF) Schizophrenia and Sex Hormones: What Is the Link?
sex videos - XVIDEOS.COM
Sex Effects - LoversLab
Sex Sex Hormone Download And Sex





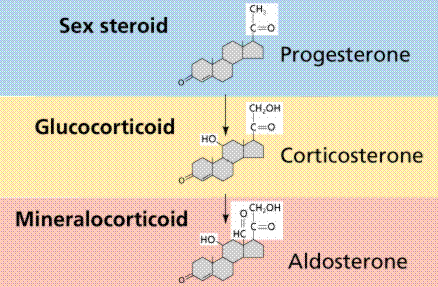
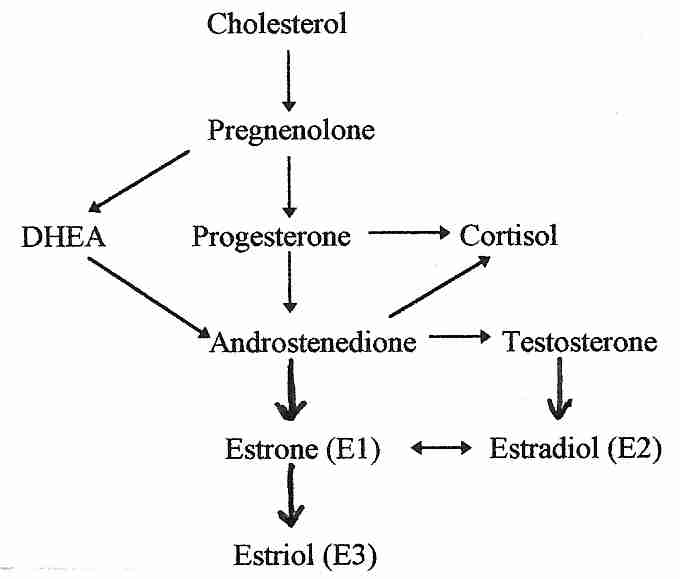
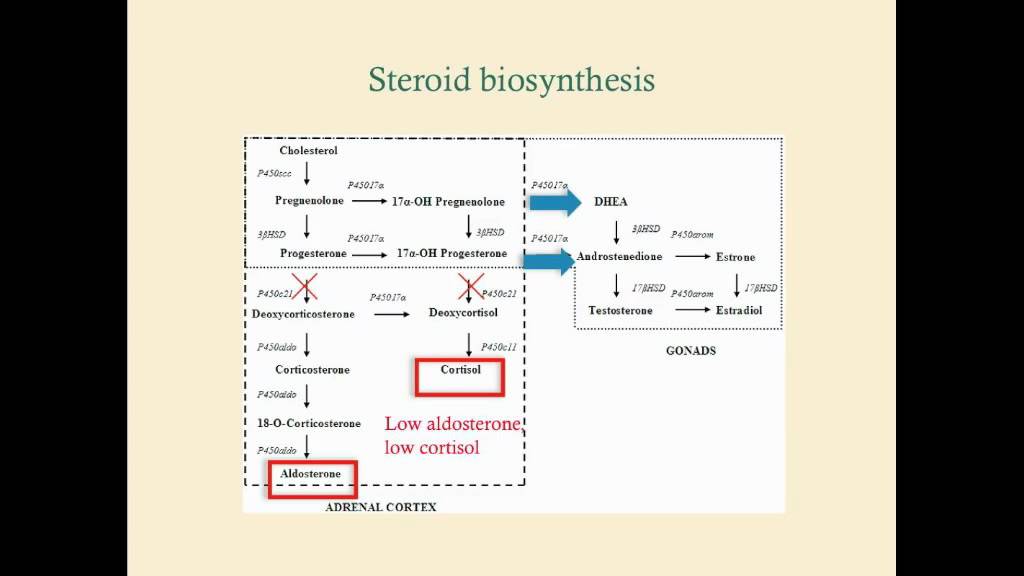
.jpg/250px-Biosinthesis_of_steroid_hormones_(simplified_version).jpg)