Rep1 German Site

👉🏻👉🏻👉🏻 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
You have reached the maximum number of saved studies (100).
Please remove one or more studies before adding more.
The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details.
ClinicalTrials.gov Identifier: NCT03496012
Last Update Posted : December 23, 2020
Information provided by (Responsible Party):
Biogen ( NightstaRx Ltd, a Biogen Company )
The objective of the study is to evaluate the efficacy and safety of a single sub-retinal injection of BIIB111 in participants with choroideremia (CHM).
This study was previously posted by NightstaRx Ltd. In October 2020, sponsorship of the trial was transferred to Biogen.
A Randomised, Open Label, Outcomes-Assessor Masked, Prospective, Parallel Controlled Group, Phase 3 Clinical Trial of Retinal Gene Therapy for Choroideremia Using an Adeno-Associated Viral Vector (AAV2) Encoding Rab Escort Protein 1 (REP1)
Participants will receive a single administration of high dose BIIB111 in one eye through sub-retinal injection after vitrectomy.
Administered as specified in the treatment arm.
Participants will receive a single administration of low dose BIIB111 in one eye through sub-retinal injection after vitrectomy.
Administered as specified in the treatment arm.
Participants will receive no sham surgery or study medication.
BCVA will be assessed for both eyes using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity (VA) chart. BCVA test should be performed prior to pupil dilation, and distance refraction should be carried out before BCVA is measured. Initially, letters are read at a distance of 4 meters from the chart. If <20 letters are read at 4 meters, testing at 1 meter should be performed. BCVA is to be reported as number of letters read correctly by the participant using the ETDRS Scale (ranging from 0 to 100 letters) in the study eye. The lower the number of letters read correctly on the eye chart, the worse the vision (or visual acuity). An increase in the number of letters read correctly means that vision has improved.
BCVA will be assessed for both eyes using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity (VA) chart. BCVA test should be performed prior to pupil dilation, and distance refraction should be carried out before BCVA is measured. Initially, letters are read at a distance of 4 meters from the chart. If <20 letters are read at 4 meters, testing at 1 meter should be performed. BCVA is to be reported as number of letters read correctly by the participant using the ETDRS Scale (ranging from 0 to 100 letters) in the study eye. The lower the number of letters read correctly on the eye chart, the worse the vision (or visual acuity). An increase in the number of letters read correctly means that vision has improved.
BCVA will be assessed for both eyes using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity (VA) chart. BCVA test should be performed prior to pupil dilation, and distance refraction should be carried out before BCVA is measured. Initially, letters are read at a distance of 4 meters from the chart. If <20 letters are read at 4 meters, testing at 1 meter should be performed. BCVA is to be reported as number of letters read correctly by the participant using the ETDRS Scale (ranging from 0 to 100 letters) in the study eye. The lower the number of letters read correctly on the eye chart, the worse the vision (or visual acuity). An increase in the number of letters read correctly means that vision has improved.
BCVA will be assessed for both eyes using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity (VA) chart. BCVA test should be performed prior to pupil dilation, and distance refraction should be carried out before BCVA is measured. Initially, letters are read at a distance of 4 meters from the chart. If <20 letters are read at 4 meters, testing at 1 meter should be performed. BCVA is to be reported as number of letters read correctly by the participant using the ETDRS Scale (ranging from 0 to 100 letters) in the study eye. The lower the number of letters read correctly on the eye chart, the worse the vision (or visual acuity). An increase in the number of letters read correctly means that vision has improved.
BCVA will be assessed for both eyes using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity (VA) chart. BCVA test should be performed prior to pupil dilation, and distance refraction should be carried out before BCVA is measured. Initially, letters are read at a distance of 4 meters from the chart. If <20 letters are read at 4 meters, testing at 1 meter should be performed. BCVA is to be reported as number of letters read correctly by the participant using the ETDRS Scale (ranging from 0 to 100 letters) in the study eye. The lower the number of letters read correctly on the eye chart, the worse the vision (or visual acuity). An increase in the number of letters read correctly means that vision has improved.
Change from baseline in contrast sensitivity in the study eye is measured using a Pelli Robson contrast sensitivity chart at 1 meter. The contrast sensitivity chart contains letters that are darkest at the top and then get progressively lighter. Scores range from 0 to 48 and are based on the number of letters read correctly. A negative change from baseline indicates a worsening in contrast sensitivity and a positive change from baseline indicates an improvement.
VFQ-25 questionnaire measures dimensions of self-reported vision-targeted health status that are most important to persons with eye disease. Total score ranges from 0-100, where a score of 0 represents the worst outcome and 100 represents the best outcome.
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
18 Years and older (Adult, Older Adult)
NOTE: Other protocol defined Inclusion/Exclusion criteria may apply.
To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor.
Please refer to this study by its ClinicalTrials.gov identifier (NCT number): NCT03496012
Los Angeles, California, United States, 90095
Miami, Florida, United States, 33136
Baltimore, Maryland, United States, 21287
New York, New York, United States, 10032
Cincinnati, Ohio, United States, 45242
Portland, Oregon, United States, 97232
Dallas, Texas, United States, 75231
Madison, Wisconsin, United States, 53705
Manchester, United Kingdom, M13 9WL
273CH301 (NSR-REP-01)
2015-003958-41 ( EudraCT Number )
Individual Participant Data (IPD) Sharing Statement:
Studies a U.S. FDA-regulated Drug Product:
Studies a U.S. FDA-regulated Device Product:
Keywords provided by Biogen ( NightstaRx Ltd, a Biogen Company ):
Choroideremia
Eye Diseases, Hereditary
Eye Diseases
Choroid Diseases
Uveal Diseases
Genetic Diseases, Inborn
Genetic Diseases, X-Linked
Sexy Young Girls Fuck
Helens Dildo
Skinny Anal Torrent
Pretty Young Girl Bad
Teens Sex Celebrity
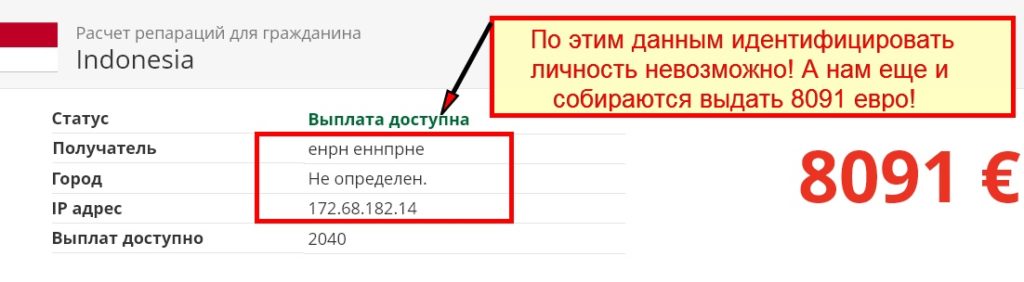
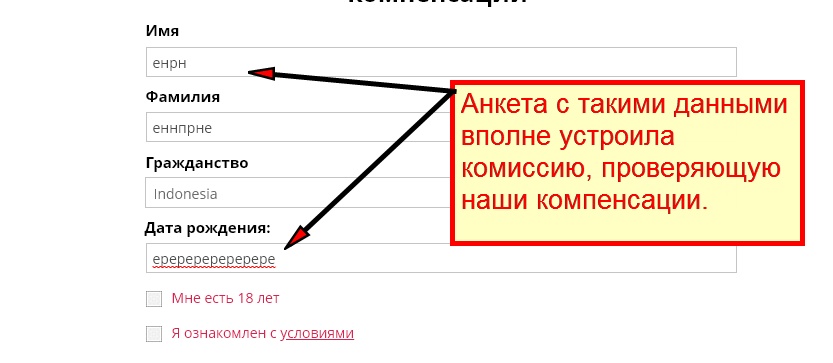
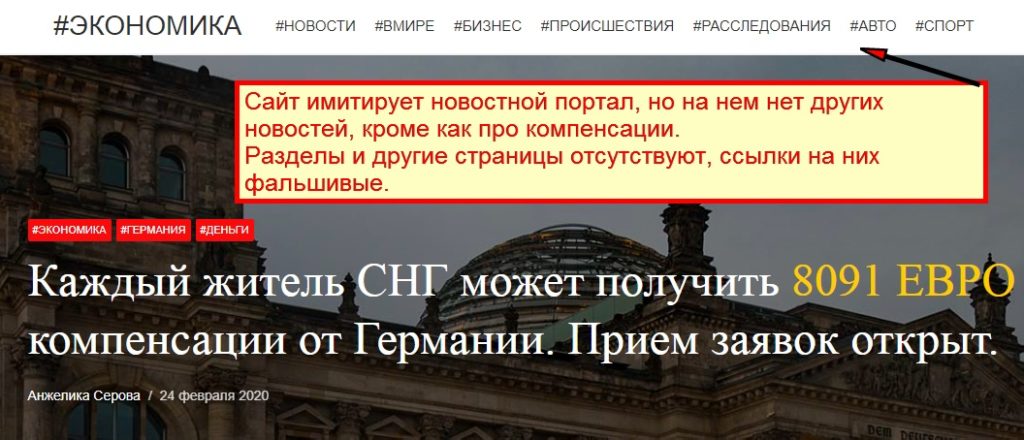
rep1-german.site Отзывы и Обзор. Развод, лохотрон или ...
Google Translate
Efficacy and Safety of BIIB111 for the Treatment of ...
Frische Einrichtungsideen und erschwingliche Möbel - IKEA ...
Long-term Safety and Efficacy Follow-up of BIIB111 for the ...
Bing Microsoft Translator
Elektronik, Autos, Mode, Sammlerstücke, Möbel und ... - eBay
AutoScout24 Europe's car market for new and used cars
BORJOMI - the deepest site in the world
Rep1 German Site