Regulation of eukaryotic cell cycle
from Khan Academia, Oxford Dictionary and Cambridge Dictionary- related proteins - belonging to the same family;
- to drive smth - to influence something or cause it to make progress;
- peaks - the point when somebody/something is best, most successful, strongest, etc.;
- partnering - to be involved in a "business" project with another "company";
- attach - to fasten or join one thing to another;
- appropriate - suitable, acceptable or correct for the particular circumstances;
- relatively - used when you are comparing something with all similar things;
- superscript - written or printed above the normal line of writing or printing;
- date back - to have existed since a particular time in the past or for the length of time mentioned;
- transition - the process or a period of changing from one state or condition to another;
- poised - completely ready for something or to do something;
- tags - anything attached to something to identify it or give information about it;
- promote - to help something to happen or develop;
- the recycle bin - a container for waste that will be recycled;
Cyclins
Cyclins are the most important core cell cycle regulators. Cyclins are a group of related proteins[1], and there are four basic types found in humans and most other eukaryotes: G_1 cyclins, G_1/S cyclins, S cyclins, and M cyclins.
As the names suggest, each cyclin is associated with a particular phase, transition, or set of phases in the cell cycle and helps drive the events[2] of that phase or period.
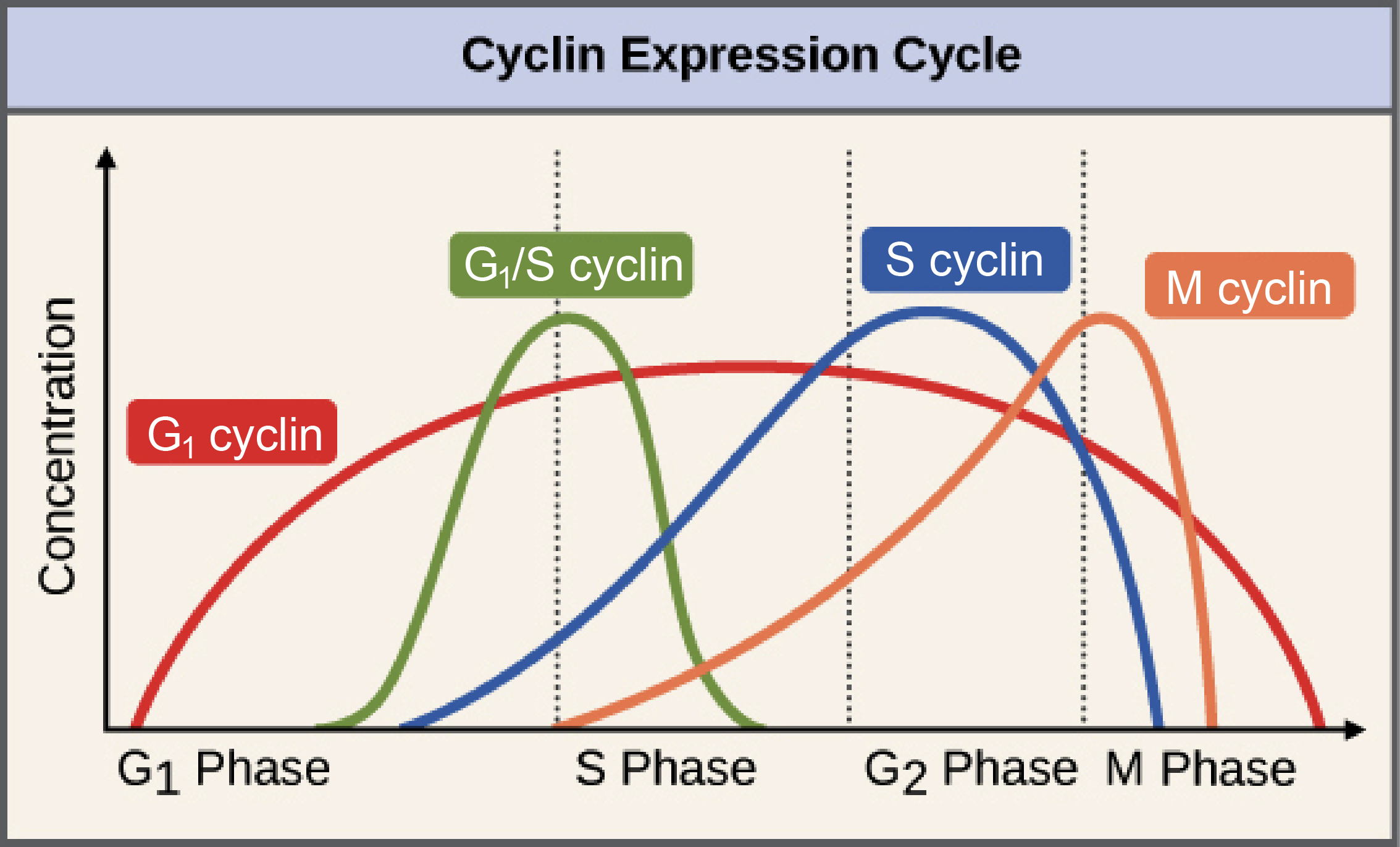
The levels of the different cyclins vary considerably across the cell cycle, as shown in the diagram. A typical cyclin is present at low levels for most of the cycle, but increases strongly at the stage where it's needed. M cyclin, for example, peaks[3] dramatically at the transition from G_2 to M phase. G_1 cyclins are unusual in that they are needed for much of the cell cycle.
Cyclin-dependent kinases
In order to drive the cell cycle forward, a cyclin must activate or inactivate many target proteins inside of the cell. Cyclins drive the events of the cell cycle by partnering[4] with a family of enzymes called the cyclin-dependent kinases (Cdks). A lone Cdk is inactive, but the binding of a cyclin activates it, making it a functional enzyme and allowing it to modify target proteins.
How does this work? Cdks are kinases, enzymes that phosphorylate (attach phosphate groups to[5]) specific target proteins. The attached phosphate group acts like a switch, making the target protein more or less active. When a cyclin attaches to a Cdk, it has two important effects: it activates the Cdk as a kinase, but it also directs the Cdk to a specific set of target proteins, ones appropriate to[6] the cell cycle period controlled by the cyclin. For instance, G_1/S cyclins send Cdks to S phase targets (e.g., promoting DNA replication), while M cyclins send Cdks to M phase targets (e.g., making the nuclear membrane break down).
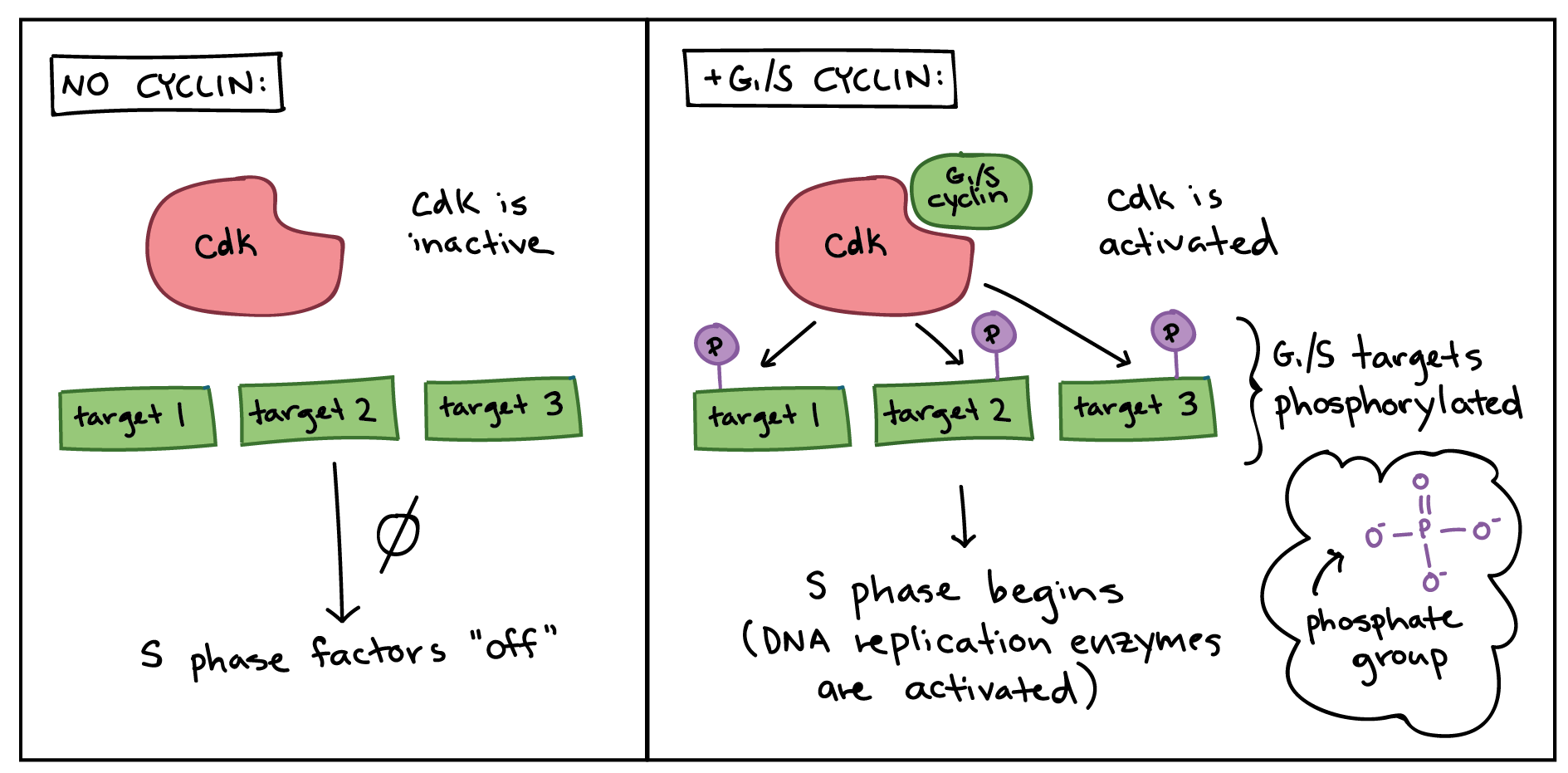
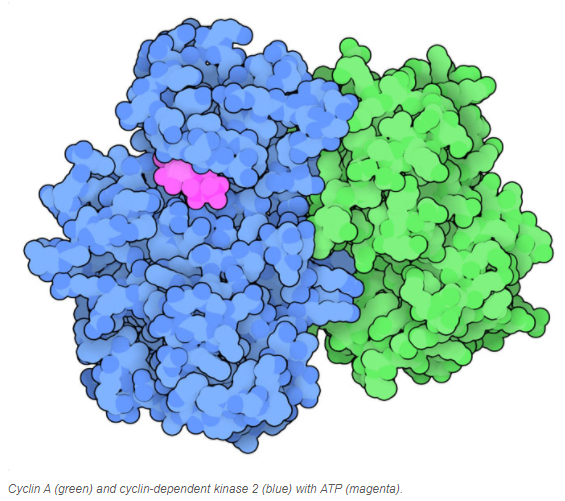
In general, Cdk levels remain relatively[7] constant across the cell cycle, but Cdk activity and target proteins change as levels of the various cyclins rise and fall. In addition to needing a cyclin partner, Cdks must also be phosphorylated on a particular site in order to be active, and may also be negatively regulated by phosphorylation of other sites, end superscript[8].
Maturation-promoting factor (MPF)
A famous example of how cyclins and Cdks work together to control cell cycle transitions is that of maturation-promoting factor (MPF). The name dates back[9] to the 1970s, when researchers found that cells in M phase contained an unknown factor that could force frog egg cells (stuck in G_2 phase) to enter M phase. This mystery molecule, called MPF, was discovered in the 1980s to be a Cdk bound to its M cyclin partner.
MPF provides a good example of how cyclins and Cdks can work together to drive a cell cycle transition[10]. Like a typical cyclin, M cyclin stays at low levels for much of the cell cycle, but builds up as the cell approaches the G_2/M transition. As M cyclin accumulates, it binds to Cdks already present in the cell, forming complexes that are poised[11] to trigger M phase. Once these complexes receive an additional signal (essentially, an all-clear confirming that the cell’s DNA is intact), they become active and set the events of M phase in motion.
The MPF complexes add phosphate tags[12] to several different proteins in the nuclear envelope, resulting in its breakdown (a key event of early M phase), and also activate targets that promote[13] chromosome condensation and other M phase events. The role of MPF in nuclear envelope breakdown is shown in simplified form in the diagram below.
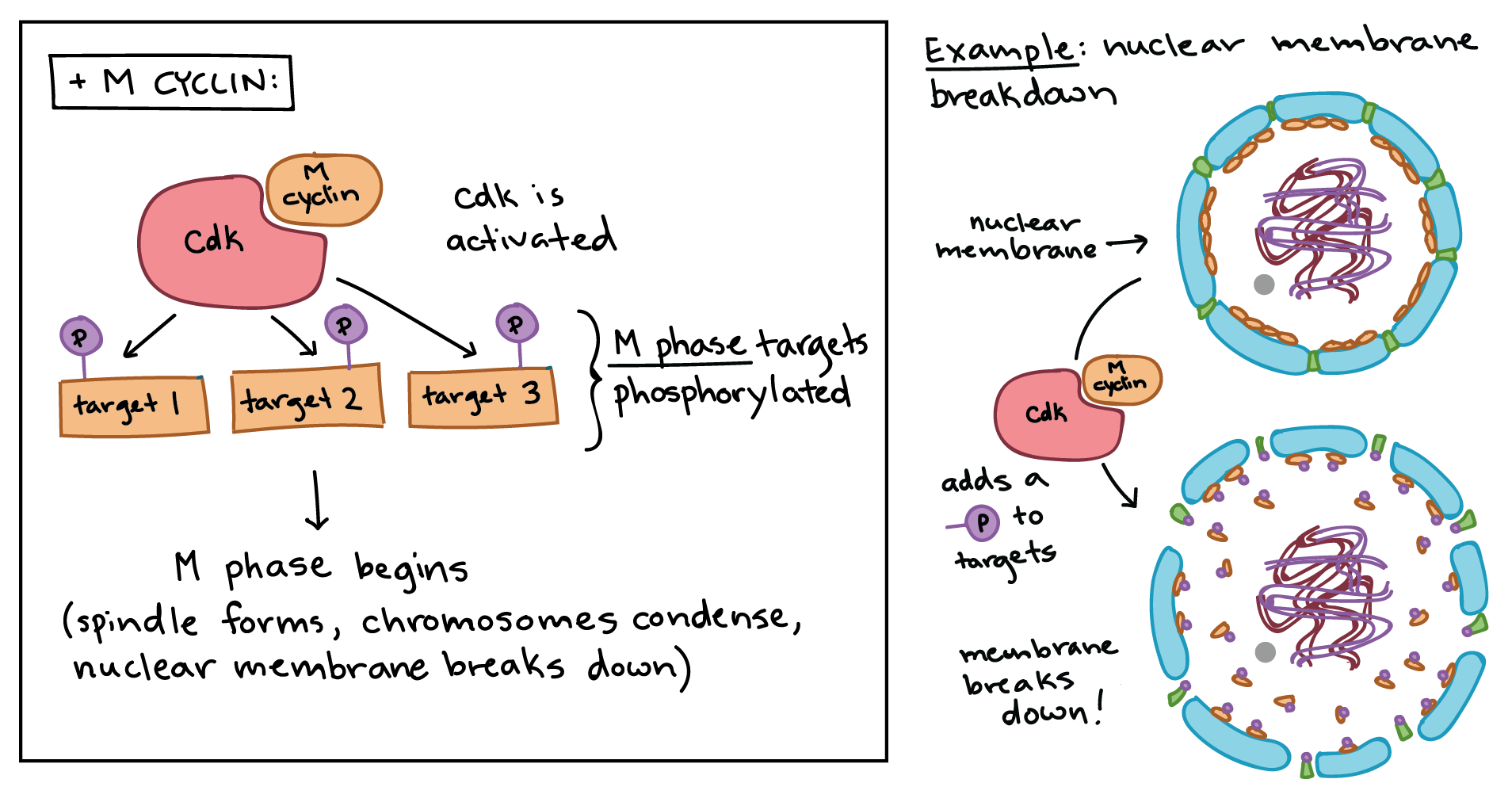
The anaphase-promoting complex/cyclosome (APC/C)
In addition to driving the events of M phase, MPF also triggers its own destruction by activating the anaphase-promoting complex/cyclosome (APC/C), a protein complex that causes M cyclins to be destroyed starting in anaphase. The destruction of M cyclins pushes the cell out of mitosis, allowing the new daughter cells to enter G_1. The APC/C also causes destruction of the proteins that hold the sister chromatids together, allowing them to separate in anaphase and move to opposite poles of the cell.
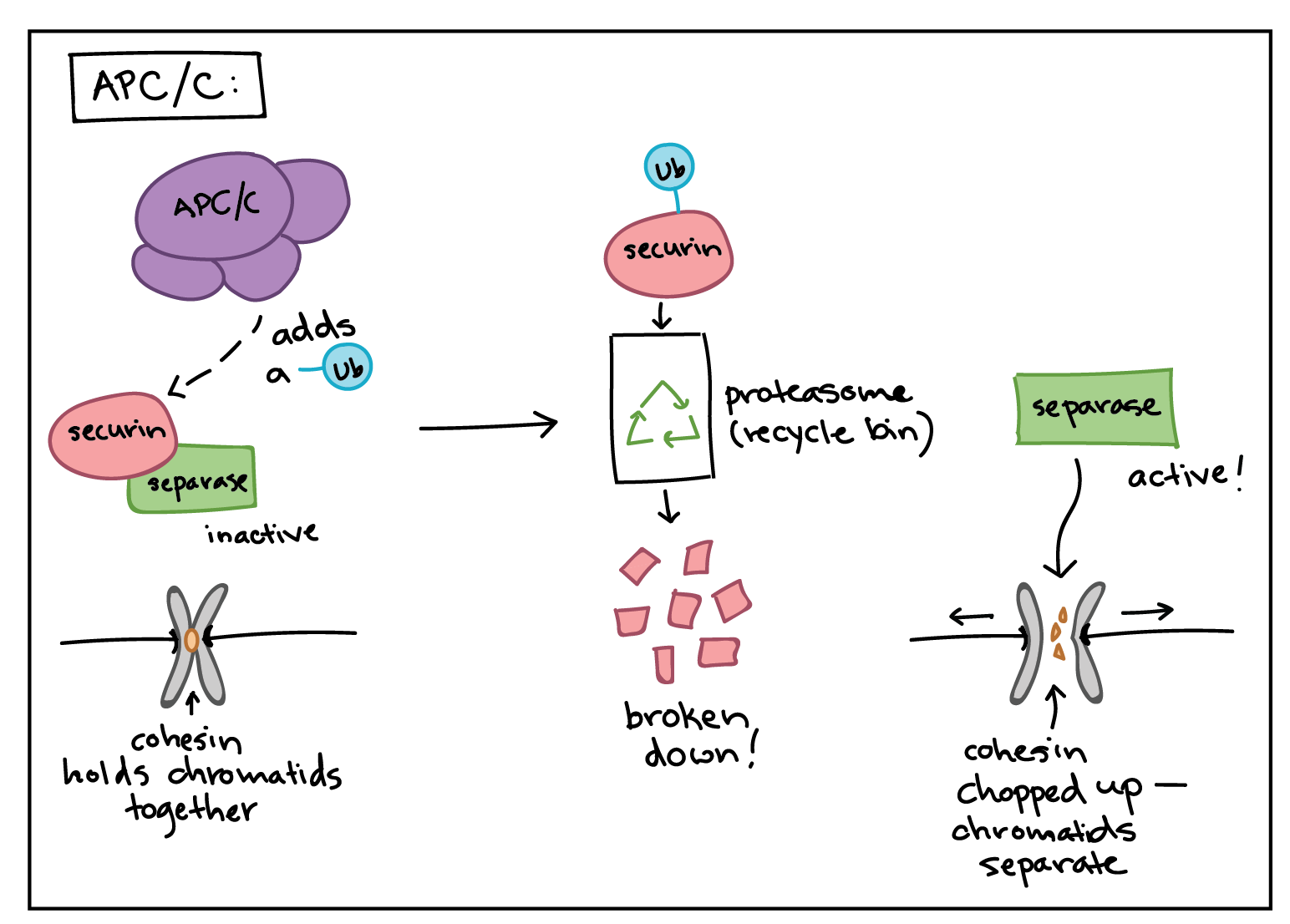
How does the APC/C do its job? Like a Cdk, the APC/C is an enzyme, but it has different type of function than a Cdk. Rather than attaching a phosphate group to its targets, it adds a small protein tag called ubiquitin (Ub). When a target is tagged with ubiquitin, it is sent to the proteasome, which can be thought of as the recycle bin[14] of the cell, and destroyed. For example, the APC/C attaches a ubiquitin tag to M cyclins, causing them to be chopped up by the proteasome and allowing the newly forming daughter cells to enter G_1.
The APC/C also uses ubiquitin tagging to trigger the separation of sister chromatids during mitosis. If the APC/C gets the right signals at metaphase, it sets off a chain of events that destroys cohesin, the protein glue that holds sister chromatids together.
- The APC/C first adds a ubiquitin tag to a protein called securin, sending it for recycling. Securin normally binds to, and inactivates, a protein called separase.
- When securin is sent for recycling, separase becomes active and can do its job. Separase chops up the cohesin that holds sister chromatids together, allowing them to separate.