Post Submission

💣 👉🏻👉🏻👉🏻 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
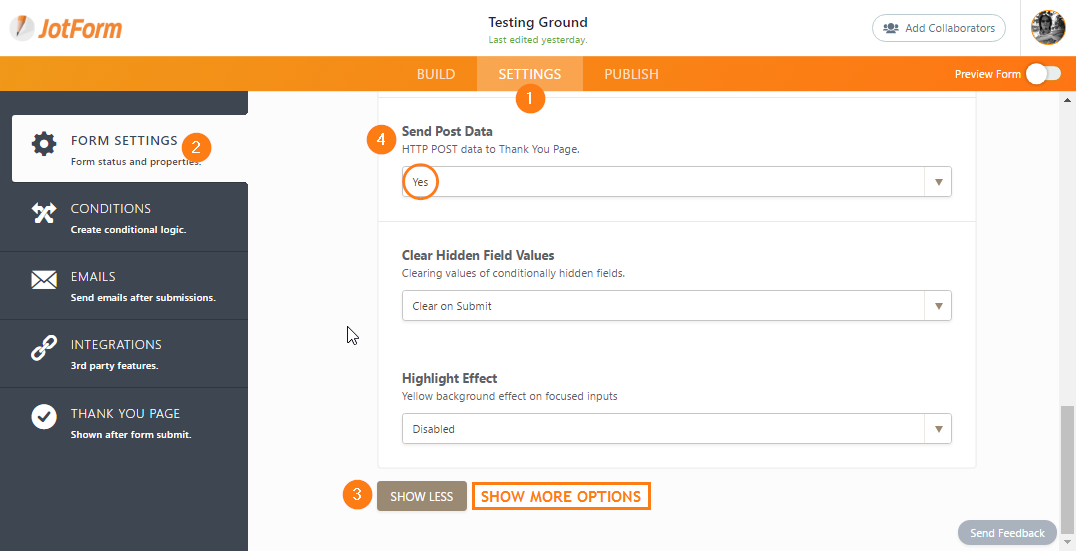
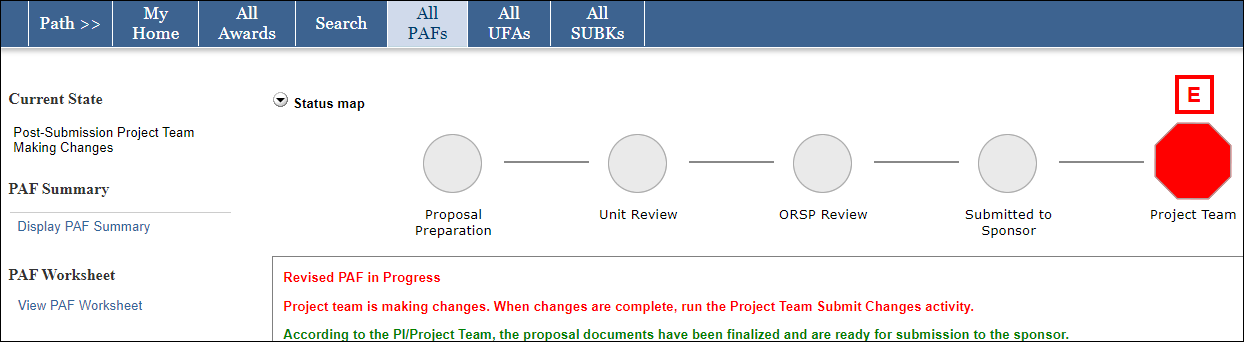
Policies, procedures and time frames vary regarding post submission materials and requirements that must be met before an award can be issued or accepted. Check sponsor guidelines for information specific to your proposal. Some University policies and procedures regarding activity after proposal submission but before an award is made are below.
An Advance Budget Number (ABN) can be set up before an award is finalized. Use the Advance Budget Number Eligibility Tool to determine if your project meets the criteria for an Advance Budget Number. If the Office of Sponsored Programs (OSP) determines it is eligible, use the SAGE “Advances” module to set up the advance budget number with GCA. Review guidance from GCA on Advance Budget Set Up & Extension.
The Department Chair or Dean is responsible for any pre-award costs that are not allowed or not funded. If this is the case, the alternative department budget number provided in your ABN request will be charged.
Your eGC1 number
Access to your eGC1
Access to Status Checker
The Request for Proposal (RFP), Program Announcement (PA) or Funding Opportunity Announcement (FOA) associated with your eGC1
IRB approval, Exempt Status Determination, or required PI assurances
IACUC approval, if applicable
Why is my eGC1 marked “ineligible” for an advance budget number (ABN)?
OSP has not yet approved the eGC1
The sponsor has not granted pre-award spending authority (most contracts and non-federal awards) and the department has not provided an alternate budget number to charge should the sponsor not reimburse costs incurred outside pre-award spending authority.
The anticipated award is a federal contract of $100,000 or more or has a subaward with federal contract funding of $3,000 or more and personnel to be charged have not been verified in the E-verify system.
Human subjects research expenditures will be charged, and IRB approval or Exempt Status determination has not been obtained.
Animal use research expenditures will be charged, and IACUC approval has not been obtained.
OSP has already prepared a funding action (FA) for the award and has sent it to GCA for processing.
SFI disclosure and review is not complete.
“Compatibility mode” of Internet Explorer (IE) may cause the tool to think you have an older version of IE than you do.
Press F12 function key to access the Developer Tools window. This displays at the bottom of the page.
Hover your mouse over the icons in the black bar at the right side of the screen and click on “Document Mode”.
In the drop-down menu, click on the choice at the top of the list – this may be “Edge”
Press the F12 key again to close the window – or use the X on the far right of the black bar.
If you are not using IE or you continue to get error messages, email web1q@uw.edu.
An advance budget number may be appropriate. They allow the next year’s advance spending to be separated so it can be properly reported.
Required training may need to be completed before the UW can accept an award.
Examples: Good Clinical Practice training, Human Subject Protections, Responsible Conduct of Research (RCR), Animal Use Medical Screening (AUMS), Biosafety, EH&S
MyResearch – Training Transcript displays records of completed training for UW’s research required training, as well as some NIH required training offered through CITI (such as Protection of Human Subject Research Participants).
MyResearch Training Transcript
Some sponsors request additional information prior to proposal review (sometimes referred to as “Supplemental Proposal Material”). This information may include items such as updated publications. Pay close attention to requests for information regarding your proposal, including instructions for who must submit materials to the sponsor.
Send all documents that need OSP review and submission, to osp@uw.edu, including your eGC1 number and PI name in the subject line.
Some sponsors request more information after the proposal has received a fundable score or review of the proposal but before making an award (sometimes referred to as “Just In Time” information). Standard requests include F&A rate agreement letters, other support information for key personnel, IRB or IACUC approval dates, and certification of required training.
Sponsor requests for this information are usually sent to both the authorized official (e.g., OSP) and the Principal Investigator. The PI prepares the documentation and provides it to OSP for review and submission to the sponsor.
Unless the Grants Management Specialist requests that JIT materials be submitted to them directly via email by the authorized official, NIH requires applicants to submit all requested information in the eRA Commons JIT section.
The NIH Policy on Genomic Data Sharing (GDS) applies to all NIH-funded research that generates large-scale human or non-human genomic data as well as the use of these data for subsequent research.
Institutional Certification cannot be provided until HSD carries out its review!
While HSD approval is pending, use the NIH Provisional Institutional Certification and include with your JIT materials. OSP can then proceed with the review and submission of those materials, pending the final certification.
When the UW is a named subrecipient under another institution’s (pass-through entity) proposal to a prime sponsor, the pass-through entity may need additional information before issuing our incoming subaward, such as a project-specific certification form or the contact information for the subaward template.
UW campus administrators typically complete these forms, using UW institutional information or information found on the UW’s FDP Expanded Clearinghouse Profile (scroll to UW profile).
If an institutional signature is required, send it to osp@uw.edu for OSP signature.
Gerberding Hall G80 Box 351202 Seattle, WA 98195
© 2021 University of Washington | Seattle, WA
www.washington.edu/research/myresearch-l…
Post Submission Materials Policy. Post-submission materials must be received by the NIH Scientific Review Officer (SRO) no later than 30 calendar days prior to the peer review meeting, unless specified otherwise in the Funding Opportunity Announcement.
public.csr.nih.gov/FAQs/ApplicantsFAQs/Po…
Where do I Send my post - submission materials?
Where do I Send my post - submission materials?
Although the post-submission materials may originate from the PD/PI, Contact PD/PI, or organizational officials, the AOR must send the materials to NIH or must send his/her concurrence to the PD/PI who will forward the materials and concurrence to NIH. A communication from the PD/PI only or with a “cc” to the AOR will not be accepted.
nexus.od.nih.gov/all/2020/09/03/how-do-i-…
When to submit post - submission materials for a grant?
When to submit post - submission materials for a grant?
Post-submission materials are those submitted after submission of the grant application but prior to initial peer review. They are not intended to correct oversights or errors discovered after submission of the application, but rather allow applicants the opportunity to respond to unforeseen events.
nexus.od.nih.gov/all/2020/09/03/how-do-i-…
When to submit post - submission materials for peer review?
When to submit post - submission materials for peer review?
Post-submission materials must be received by the SRO no later than 30 calendar days prior to the peer review meeting. Post-submission materials will not be accepted after this time, unless specifically allowed in the FOA for which the application was submitted or in a special Guide Notice.
nexus.od.nih.gov/all/2020/09/03/how-do-i-…
https://www.linguee.com/english-russian/translation/post+submission.html
Many translated example sentences containing "post submission" – Russian-English dictionary and search engine for Russian translations.
https://www.washington.edu/.../plan-and-propose/submit-proposal/post-submission
Перевести · 19.03.2021 · Post Submission Contents. Policies, procedures and time frames vary regarding post submission materials and requirements that must be... Advance Budget …
Post Submission, from Approved to Funded
YouTube › Newton Connectivity Systems
How to Allow Post Submissions From the Frontend
YouTube › WPForms - WordPress Forms Plugin
jet engine custom content types. & front-end post submission
HOW TO POST YOUR SUBMISSION IN A DISCUSSION FORUM
YouTube › MUELE Videos for Students
Frontend Post Submission by Users in WordPress with Elementor and Dynamic Content Plugin
https://public.csr.nih.gov/FAQs/ApplicantsFAQs/PostSubmissionMaterialsPolicy
Перевести · Allowable Post-Submission Materials are listed in the NIH Policy NOT-OD-19-083. Post-submission materials must be received by the NIH Scientific Review Officer (SRO) no …
https://www.lawinsider.com/dictionary/post-submission-period
Перевести · Define Post-Submission Period. means the period of time from the Submission Deadline to the Closing Date.
https://nexus.od.nih.gov/all/2020/09/03/how-do-i-submit-post-submission-materials-for...
Перевести · 03.09.2020 · Post-submission materials are those submitted after submission of the grant application but prior to initial peer review. They are not intended to correct oversights or errors discovered after submission …
https://stackoverflow.com/questions/133925
Перевести · 24.09.2008 · For example, post_to_url("http://google.com/",{ submit: "submit" } );. I have patched the function slightly to walk around this variable space collision. function post_to_url(path, params, method) { method = method || "post"; var form = document.createElement("form"); //Move the submit …
Dynamically create s in a form and submit it /** * sends a request to the specified url from a form. this will change the window location...
This would be a version of the selected answer using jQuery . // Post to the provided URL with the specified parameters. function post(path, param...
A simple quick-and-dirty implementation of @Aaron answer: document.body.innerHTML += 'Naruto Xxx Parody
Sexy Videos Mom And Son Milf Brazzers
New Video Boys Omegle
Elise Erotic Pantyhose
Latex Slut Hogtied
post submission - Russian translation – Linguee
Post Submission - UW Research
Post Submission Materials Policy | NIH Center for ...
Post-Submission Period | legal definition of Post ...
How Do I Submit Post-Submission Materials for My ...
Guest Post Submission - IoT For All
List of 200+ Sites for Submit guest post || Blogili
Guest post submissions | VentureBeat
Post Submission














_December2012.png)












.png)