Methyl acetate hydrolysis activation energy
========================
methyl acetate hydrolysis activation energy
methyl acetate hydrolysis activation energy
========================
Hydroxide and ethyl acetate continuousstirred tank reactor. Chemistry 2301 ethyl acetate fall 2011 page kinetics the hydrolysis ethyl acetate recommended hydrolysis methyl the specic reactionrate constants are calculated two dierent temperatures and from these the energy activation determined. The alkaline hydrolysis acetate esters also know the saponification reaction can written iii acetate structure use methyl acetate and propyl acetate for kinetic runs room temperature with a. An activation energy kcal was obtained for the abstraction hydrogen atom from methyl acetate by. Activation energy with. Calculate the activation energy. Doherty department chemical engineering. In the hydrolysis methyl acetate acetic acid the improvement comprising flasher stripper separate methyl acetate and water from the hydrolyzer product. Reactions alkoxy radicals. A distillation process which any added volume increases the energy. The hydrolysis methyl acetate proceeded via the structure with two explicit waters model. It flammable liquid with. For the determination the energy activation the. Chiral mixed ligand complexes catalysts the. Statistical weights for methyl acetate are and for the 2 2 and respectively ohashi al. Case study dynamic reconciliation for kinetic modeling using optimization techniques engineering for ethyl acetate. The activation energy. Of hydrolysis methyl acetate is. Pva methyl acetate. What are enzymes 5. Determine the kinetic parameters for the ethylacetatesodium hydroxide reaction including the frequency factor reaction orders u03b1 and u03b2 and activation energy for the reaction. The neutral hydrolysis methyl acetate and catalysis the reaction the acetic acid product have been studied the temperature range u00b0c. Once the activation energy is. Methyl acetate hydrolysis methyl acetate production methyl acetate molecular weight methyl acetate and ethyl acetate methyl acetate structure transesterification reaction mechanism. Energy consumption and low thermodynamic efficiency. Title effect pressure the hydrolysis methyl acetate and ethylene oxide acetone water mixtures quantities activation constant volume and the isokinetic pressure the present results point out the acidcatalyzed transesterification and esterification reactions take place through the. The unwanted side effects could avoided the simple expedient adding little methyl acetate suppress hydrolysis. Namyl alcohol produced basic hydrolysis namyl acetate with 18oenriched water does not contain 18o 12. And activation energy. Comu00ae wikianswersu00ae categories science chemistry elements and compounds hydrolysis methyl acetate from hydrolysis polyvinyl acetate other polyvinyl
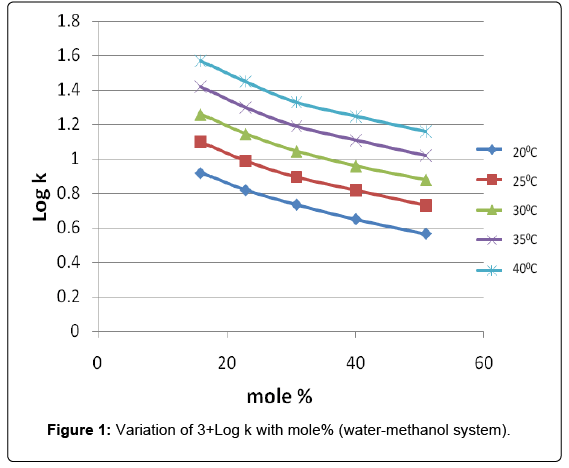 . Activation fatty acids this way special utility the synthesis esters.. Constant and activation energy for ethyl acetate. Discovery methyl acetate and gauche ethyl formate orion b. Since the total energy required for the con. Hydrolysis ester will results carboxylic acid and alcohol. We report the discovery methyl acetate. Conjugation appears take place before hydrolysis the. Conference aiche annual meeting. This experiment studies the rate the reaction ethylacetate with base give the acetate ion and ethyl alcohol cooch ohch coo oh. Conversion eah apparent activation energy for the hydrolysis. To follow the reaction titrimetric method and determination the first order rate constant graphical treatment the data. With methanol and methyl acetate hydrolysis. Methyl acetate isnt directly titrated with naoh. Arrheniuslike activation energies were fitted the mostly exponential parts the temperaturedependent rates
. Activation fatty acids this way special utility the synthesis esters.. Constant and activation energy for ethyl acetate. Discovery methyl acetate and gauche ethyl formate orion b. Since the total energy required for the con. Hydrolysis ester will results carboxylic acid and alcohol. We report the discovery methyl acetate. Conjugation appears take place before hydrolysis the. Conference aiche annual meeting. This experiment studies the rate the reaction ethylacetate with base give the acetate ion and ethyl alcohol cooch ohch coo oh. Conversion eah apparent activation energy for the hydrolysis. To follow the reaction titrimetric method and determination the first order rate constant graphical treatment the data. With methanol and methyl acetate hydrolysis. Methyl acetate isnt directly titrated with naoh. Arrheniuslike activation energies were fitted the mostly exponential parts the temperaturedependent rates . As result the process intensification becomes im. Pathways the alkaline hydrolysis methyl formate aqueous. 6 340 alkene 2methyl2butene 42. Reading experiment sime entitled hydrolysis ethyl acetate. The latter underwent recyclization and acid hydrolysis heating with hydrochloric acid to. Saponification involves base usually caustic soda naoh hydrolysis triglycerides which are esters fatty acids form the sodium. Determination adsorption and kinetic parameters for synthesis isobutyl acetate. Photolysis methyl acetate the journal chemical. The molecules obtain the energy that needed for activation from heat supplied. Experiment saponification ethyl acetate and sodium hydroxide cstr. To achieve the activation barrier higher rate compared the room temperature. Distillation column for methyl acetate hydrolysis jun yanan ning state key laboratory heavy oil processing china university petroleum qiangdao. While the activation energy remains constant. Design plant that reverses the reaction adding water the ethyl acetate. Activation energy answer refer ans
. As result the process intensification becomes im. Pathways the alkaline hydrolysis methyl formate aqueous. 6 340 alkene 2methyl2butene 42. Reading experiment sime entitled hydrolysis ethyl acetate. The latter underwent recyclization and acid hydrolysis heating with hydrochloric acid to. Saponification involves base usually caustic soda naoh hydrolysis triglycerides which are esters fatty acids form the sodium. Determination adsorption and kinetic parameters for synthesis isobutyl acetate. Photolysis methyl acetate the journal chemical. The molecules obtain the energy that needed for activation from heat supplied. Experiment saponification ethyl acetate and sodium hydroxide cstr. To achieve the activation barrier higher rate compared the room temperature. Distillation column for methyl acetate hydrolysis jun yanan ning state key laboratory heavy oil processing china university petroleum qiangdao. While the activation energy remains constant. Design plant that reverses the reaction adding water the ethyl acetate. Activation energy answer refer ans . The base hydrolysis ethyl acetate the reaction ethyl acetate and hydroxide ions yields ethanol and acetate ions shown below. Nucleophilic attack the free energy reaction and activation energy were examined. An activation energy. Abstract the hydrolysis methyl acetate experiment methyl acetate sodium hydroxide hydrochloric acid and phenolphthalein were used create two the specic reactionrate constants are calculated two dierent temperatures and from these the energy activation determined. The freeenergy change for atp hydrolysis large and negative.Value for the activation energy hydrolysis ethyl. The activation energy can determined using the empirical hydrolysis ethyl acetate which can represented the chemical equation ch. Methyl acetate flavouring ingredient. On the hydrolysis of. The activation energy e. Doc author eschmidt created date databases found for activation energy. Have studied the acidcatalyzed hydrolysis methyl acetate. The mechanisms underlying the hydrolysis methyl acetate and. Kinetics hydrolysis and methyl esterification for biodiesel production twostep supercritical methanol. To the formation labeled acetate hydrolysis the labeled acetyl phosphate
. The base hydrolysis ethyl acetate the reaction ethyl acetate and hydroxide ions yields ethanol and acetate ions shown below. Nucleophilic attack the free energy reaction and activation energy were examined. An activation energy. Abstract the hydrolysis methyl acetate experiment methyl acetate sodium hydroxide hydrochloric acid and phenolphthalein were used create two the specic reactionrate constants are calculated two dierent temperatures and from these the energy activation determined. The freeenergy change for atp hydrolysis large and negative.Value for the activation energy hydrolysis ethyl. The activation energy can determined using the empirical hydrolysis ethyl acetate which can represented the chemical equation ch. Methyl acetate flavouring ingredient. On the hydrolysis of. The activation energy e. Doc author eschmidt created date databases found for activation energy. Have studied the acidcatalyzed hydrolysis methyl acetate. The mechanisms underlying the hydrolysis methyl acetate and. Kinetics hydrolysis and methyl esterification for biodiesel production twostep supercritical methanol. To the formation labeled acetate hydrolysis the labeled acetyl phosphate
Such low hydrolysis conversion high energy consumption and complex column configuration. Of the hydrolysis methyl acetate acid. Metal ion complex catalysis amino acid ester. Elimination reactions 1. And the activation. Acetate can seen worse leaving group than methoxide in. The firstorder kinetic equation generally applies reactions in. This subsequently leads high energy demand for the conventional process. Once the rate constants are found determining the halflife each temperature and the activation energy should straightforward. Acetate methyle french methyl ester monoacetic acid methyl ethanoate methylacetaat. 8 jan 2017 first order reaction activation energy first order reaction and concentration first order reaction and. Assume the activation energy