LIQUID GLASS
https://search.aepiot.ro/search.html?q=LIQUID%20GLASSGo

GlassGlass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability into any shape, glass has been traditionally used for vessels, such as bowls, vases, bottles, jars and drinking glasses. Soda–lime glass, containing around 70% silica, accounts for around 90% of modern manufactured glass. Glass can be coloured by adding metal salts or painted and printed with vitreous enamels, leading to its use in stained glass windows and other glass art objects. The refractive, reflective and transmission properties of glass make glass suitable for manufacturing optical lenses, prisms, and optoelectronics materials. Extruded glass fibres have applications as optical fibres in communications networks, thermal insulating material when matted as glass wool to trap air, or in glass-fibre reinforced plastic (fibreglass).
In connection with: Glass
Description combos: panes Glass is other with which vitreous using bottles
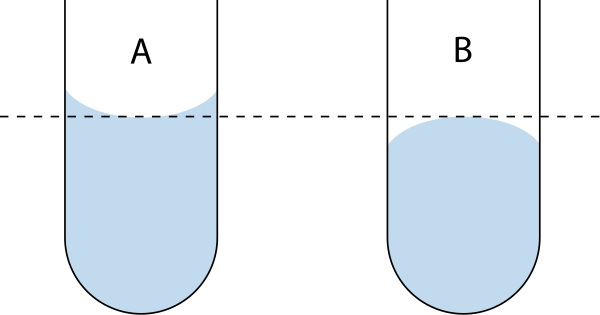
Meniscus (liquid)In physics (particularly liquid statics), the meniscus (pl.: menisci, from Greek 'crescent') is the curve in the upper surface of a liquid close to the surface of the container or another object, produced by surface tension. A concave meniscus occurs when the attraction between the particles of the liquid and the container (adhesion) is more than half the attraction of the particles of the liquid to each other (cohesion), causing the liquid to climb the walls of the container (see Surface tension § Causes). This occurs between water and glass. Water-based fluids like sap, honey, and milk also have a concave meniscus in glass or other wettable containers. Conversely, a convex meniscus occurs when the adhesion energy is less than half the cohesion energy. Convex menisci occur, for example, between mercury and glass in barometers and thermometers. In general, the shape of the surface of a liquid can be complex. For a sufficiently narrow tube with circular cross-section, the shape of the meniscus will approximate a section of a spherical surface, while for a large container, most of the upper surface of the liquid will be almost flat, only curving up (if concave) or down (if convex) near the edges.
In connection with: Meniscus (liquid)
Title combos: liquid Meniscus
Description combos: other the concave complex the energy another meniscus the

Smart glassSmart glass, also known as switchable glass, dynamic glass, and smart-tinting glass, is a type of glass that can change its optical properties, becoming opaque or tinted, in response to electrical or thermal signals. This can be used to prevent sunlight and heat from entering a building during hot days, improving energy efficiency. It can also be used to conveniently provide privacy or visibility to a room. There are two primary classifications of smart glass: active or passive. The most common active glass technologies used today are electrochromic, liquid crystal, and suspended particle devices (SPD). Thermochromic and photochromic are classified as passive technologies. When installed in the envelope of buildings, smart glass helps to create climate adaptive building shells, which benefits include things such as natural light adjustment, visual comfort, UV and infrared blocking, reduced energy use, thermal comfort, resistance to extreme weather conditions, and privacy. Some smart windows can self-adapt to heat or cool for energy conservation in buildings. Smart windows can eliminate the need for blinds, shades or window treatments. Some effects can be obtained by laminating smart film or switchable film onto flat surfaces using glass, acrylic or polycarbonate laminates. Some types of smart films can be applied to existing glass windows using either a self-adhesive smart film or special glue. Spray-on methods for applying clear coatings to block heat and conduct electricity are also under development.
In connection with: Smart glass
Title combos: glass Smart
Description combos: When classified of SPD glass as development methods either

Storm glassThe storm glass or chemical weather glass was an instrument claimed to help predict weather. It consists of a special liquid placed inside a sealed transparent glass. The state of crystallization within the liquid was believed to be related to the weather. The inventor is unknown but the device became popular in the 1860s after being promoted by Royal Navy Admiral Robert FitzRoy who claimed that "if fixed, undisturbed, in free air, not exposed to radiation, fire, or sun, but in the ordinary light of a well-ventilated room or outer air, the chemical mixture in a so-called storm-glass varies in character with the direction of the wind, not its force, specially (though it may so vary in appearance only) from another cause, electrical tension". The compositions of the liquid in a storm glass varies but usually contains "camphor, nitrate of potassium and sal-ammoniac, dissolved by alcohol, with water and some air." These devices are now known to have little value in weather prediction, and tend to change visually based on the surrounding temperature, however they do not react to pressure changes and continue to be a curiosity.
In connection with: Storm glass
Title combos: Storm glass
Description combos: glass some have in cause related popular but within

Glass transitionThe glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists, because the glass is a higher energy state (or enthalpy at constant pressure) than the corresponding crystal. Hard plastics like polystyrene and poly(methyl methacrylate) are used well below their glass transition temperatures, i.e., when they are in their glassy state. Their Tg values are both at around 100 °C (212 °F). Rubber elastomers like polyisoprene and polyisobutylene are used above their Tg, that is, in the rubbery state, where they are soft and flexible; crosslinking prevents free flow of their molecules, thus endowing rubber with a set shape at room temperature (as opposed to a viscous liquid). Despite the change in the physical properties of a material through its glass transition, the transition is not considered a phase transition; rather it is a phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 kelvins per minute (36 °F/min)) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. The question of whether some phase transition underlies the glass transition is a matter of ongoing research.
In connection with: Glass transition
Title combos: Glass transition
Description combos: than well Tg the 212 Tm crystalline properties extending
Viscous liquidIn condensed matter physics and physical chemistry, the terms viscous liquid, supercooled liquid, and glass forming liquid are often used interchangeably to designate liquids that are at the same time highly viscous (see Viscosity of amorphous materials), can be or are supercooled, and able to form a glass.
In connection with: Viscous liquid
Title combos: liquid Viscous
Description combos: viscous same form are same In highly and to
Liquid GlassLiquid Glass is a graphical user interface and design language developed by Apple as a unified visual theme across its iOS, iPadOS, macOS, watchOS, tvOS, and visionOS operating systems. It was first announced on June 9, 2025, at the 7th annual Worldwide Developers Conference (WWDC). Liquid Glass features a more fluid and glass-like interface being introduced in iOS 26, iPadOS 26, macOS Tahoe, tvOS 26, and watchOS 26.
In connection with: Liquid Glass
Title combos: Liquid Glass
Description combos: design It interface Apple by and Glass interface 2025
Quick Access
Tag Explorer
Discover Fresh Ideas in the Universe of aéPiot
MultiSearch | Search | Tag Explorer
SHEET MUSIC | DIGITAL DOWNLOADS