In Autosomal Dominant Inheritance Asp Detid

👉🏻👉🏻👉🏻 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
Don't delay your care at Mayo Clinic
Our general interest e-newsletter keeps you up to date on a wide variety of health topics.
In an autosomal dominant disorder, the mutated gene is a dominant gene located on one of the nonsex chromosomes (autosomes). You need only one mutated gene to be affected by this type of disorder. A person with an autosomal dominant disorder — in this case, the father — has a 50% chance of having an affected child with one mutated gene (dominant gene) and a 50% chance of having an unaffected child with two normal genes (recessive genes).
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic.
Our general interest e-newsletter keeps you up to date on a wide variety of health topics.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Autosomal dominant inheritance refers to a mutation on one of the 22 pairs of nuclear chromosomes (i.e. non-sex chromosomes) that leads to syndrome expression when only one copy of the chromosome pair carries the mutant allele.
Alan Lap-Yin Pang, Wai-Yee Chan, in Essential Concepts in Molecular Pathology, 2010
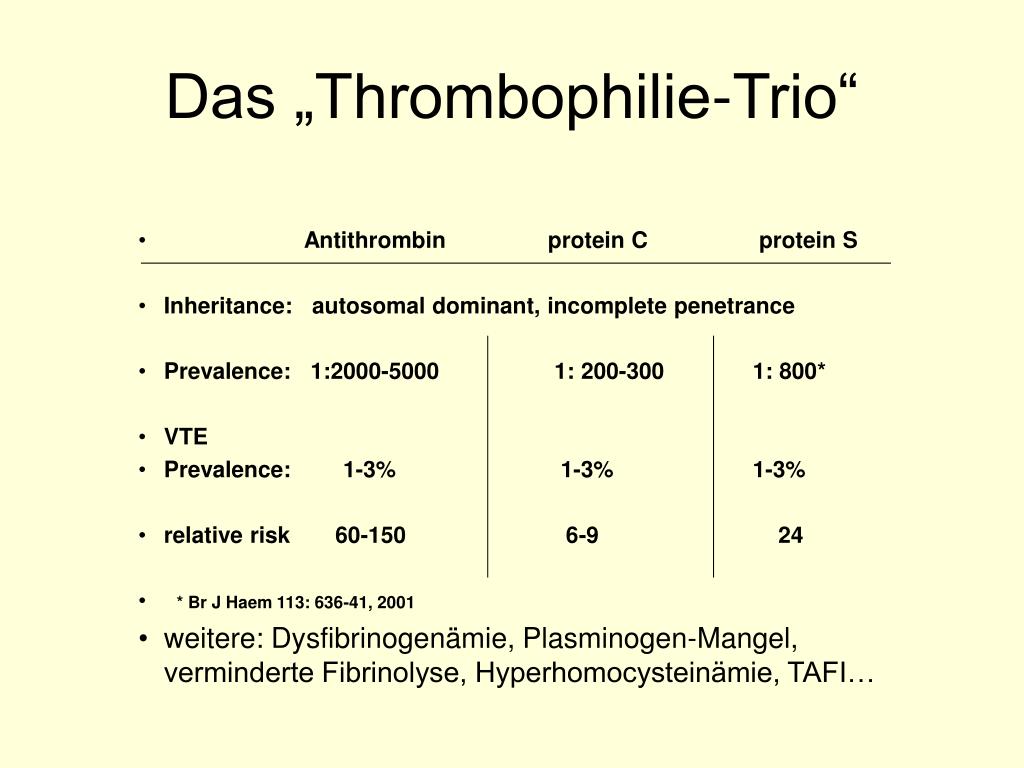
ADH is a familial form of isolated hypoparathyroidism characterized by hypocalcemia, hyperphosphatemia, and normal to hypoparathyroidism. Inheritance of the disorder follows an autosomal dominant mode. The patients are generally asymptomatic. A significant fraction of cases of idiopathic hypoparathyroidism may in fact be ADH.
More than 80% of the reported ADH kindreds have CaSR mutations. There are 44 activating mutations of CaSR reported that produce a gain of CaSR function when expressed in in vitro systems. The majority of the ADH mutations are missense mutations within the extracellular domain and transmembrane domain of CaSR. The mechanism of CaSR activation by these mutations is not known. Interestingly, almost every ADH family has its own unique missense heterozygous CaSR mutation. Most ADH patients are heterozygous. The only deletion-activating mutation occurs in a homozygous patient in an ADH family. However, there is no apparent difference in the severity of the phenotype between heterozygous and homozygous patients.
URL: https://www.sciencedirect.com/science/article/pii/B9780123744180000220
Alan Lap-Yin Pang, ... Wai-Yee Chan, in Molecular Pathology, 2009
ADH is a familial form of isolated hypoparathyroidism characterized by hypocalcemia, hyperphosphatemia, and normal to hypoparathyroidism. Inheritance of the disorder follows an autosomal dominant mode. The patients are generally asymptomatic. A significant fraction of cases of idiopathic hypoparathyroidism may in fact be ADH.
More than 80% of the reported ADH kindreds have CaSR mutations. There are 44 activating mutations of CaSR reported in the literature. These mutations produce a gain of CaSR function when expressed in in vitro systems [13,117]. The majority of the ADH mutations are missense mutations within the extracellular domain and transmembrane domain of CaSR. In addition, a deletion in the intracellular domain, p.S895_V1075del, has also been described in an ADH family. The mechanism of CaSR activation by these mutations is not known. Worthwhile noting is that almost every ADH family has its own unique missense heterozygous CaSR mutation [117]. Most ADH patients are heterozygous. The only deletion-activating mutation occurs in a homozygous patient in an ADH family. However, there is no apparent difference in the severity of the phenotype between heterozygous and homozygous patients.
URL: https://www.sciencedirect.com/science/article/pii/B9780123744197000226
Guy Helman, ... Adeline Vanderver, in Handbook of Clinical Neurology, 2018
ADLD is caused by heterozygous duplications in the gene encoding Lamin B1 (LMNB1) (150340) (Padiath et al., 2006; Giorgio et al., 2015). Inheritance is autosomal dominant. Apparent sporadic cases are reported in addition to cases of autosomal-dominant inheritance. Deletion/duplication analysis of LMNB1 should be performed by gene sequencing, either by single-gene testing or as part of a multigene panel, or can be tested by chromosomal microarray or polymerase chain reaction-based methods (Nahhas et al., 1993).
URL: https://www.sciencedirect.com/science/article/pii/B9780444640765000430
Isolated autosomal dominant PLD (ADPLD) (MIM 174050) also occurs as a genetically distinct disease in the absence of renal cysts (274, 281, 328). Like ADPKD, ADPLD is genetically heterogeneous, with two genes identified (PRKCSH and SEC63) accounting for approximately one third of isolated ADPLD cases (49, 57, 166). ADPLD often goes undetected even in first-degree relatives of patients with highly symptomatic polycystic liver disease. As in the case of polycystic liver disease associated with ADPKD, isolated ADPLD is more severe in women than in men. Liver function tests remain normal and when symptoms develop, these are related to mass effects or complications such as cyst hemorrhage or infection. Patients with isolated ADPLD may also be at increased risk for intracranial aneurysms and valvular heart disease (274).
URL: https://www.sciencedirect.com/science/article/pii/B978012088488950084X
Elliott H. Sohn, ... Edwin M. Stone, in Retina (Fifth Edition), 2013
ADVIRC was first described by Kaufman et al. in 198273 as a condition with: (1) an autosomal dominant inheritance pattern; (2) peripheral pigmentary retinopathy for 360 degrees, with a discrete posterior boundary near the equator (see Fig. 42.9); (3) punctate whitish opacities in the retina; (4) vitreous cells and fibrillar condensation; (5) blood–retinal barrier breakdown; (6) retinal arteriolar narrowing and occlusion; (7) retinal neovascularization; (8) choroidal atrophy; and (9) presenile cataracts (see Fig. 42.9).74 The EOG is usually abnormal with a relatively normal ERG,75 but the first electrophysiologic studies of ADVIRC patients27,75,76 occurred in the pre-molecular era when genetic testing was not available. It has since been discovered that ADVIRC is caused by splice-altering mutations in BEST1 and that these patients can also have concomitant developmental abnormalities, including microcornea, hyperopia, and shortened axial length.26,77,78 Some patients have a severe form of ADVIRC in which both the ERG and EOG are abnormal, thus resembling retinitis pigmentosa.79,80
URL: https://www.sciencedirect.com/science/article/pii/B9781455707379000424
Autosomal dominant epilepsy with auditory features (ADEAF), also known as autosomal dominant lateral temporal lobe epilepsy (ADLTLE), is a rare epilepsy syndrome that confers focal seizures that often secondarily generalize.91 The seizures can begin at any age, but usually start in the second or third decade of life. As the syndrome’s name suggests, the majority of the patients (64%) have focal seizures that begin with an auditory component. Some have simple auditory hallucinations, such as ringing that changes in volume,92 or a “buzzing, or humming like a machine.” 93 In other patients, auditory hallucinations are formed, such as voices or singing.92 Some patients have visual, autonomic, psychic or vertiginous focal seizures. In some pedigrees, the seizures are accompanied by a sensory aphasia with or without auditory hallucinations.94 In most patients, the seizures are infrequent (only several times per year before starting medication) and can usually be controlled with anticonvulsant drugs.
Interictal EEG abnormalities, if present, are usually left temporal spike and sharp wave complexes. ADEAF patients do not have causative brain lesions on conventional MRI imaging. However, one diffusion tensor imaging study suggested that some patients may have subtle malformations in the left temporal cortex.95 Finally, although their neurological exams are normal, functional imaging and magnetoencephalography studies of members of four ADEAF families were consistent with impaired language processing.96
At the time of its first description, ADEAF was linked to a 10-cM region on chromosome 10q with a 71% penetrance.97 Linkage studies in another family narrowed the region to approximately 3 cM.93 Kalachikov et al. sequenced all exons and intron/exon junctions from one affected patient form three different ADEAF pedigrees and then genotyped all family members from five different ADEAF pedigrees.98 They found that all affected family members and obligate carriers possessed mutations (four frameshift/intron retention truncation mutations and one missense mutation) in the leucine-rich, glioma-inactivated 1 gene (LGI1). Some unaffected family members also possessed the mutations, a finding consistent with the reduced penetrance found in the gene linkage studies. There are now 27 LGI1 mutations associated with ADEAF (Table 84.4).
Less than 50% of ADEAF families and less than 2% of sporadic ADEAF patients have LGI1 mutations. Recently, a new ADEAF locus was found in a large Brazilian family. The DNA from 11 affected and 14 unaffected family and performed genotyping found linkage to region 19q13.11–q13.31 with incomplete penetrance.99
URL: https://www.sciencedirect.com/science/article/pii/B978012410529400084X
ADO II (Albers-Schönberg disease) is characterized by generalized osteosclerosis with thickening of the vertebral end plates (i.e., sandwich vertebrae) and bone within bone in the pelvis. Although ADO II has been called benign osteoporosis, most patients have clinical problems, presumably related to impaired bone remodeling, that include fractures, osteoarthritis, skeletal deformities, and cranial nerve involvement.533 Most patients with ADO II have a milder form of mutation in the chloride transport gene ClCN7 than that seen in the more severe forms of infantile osteopetrosis, although other genes may be involved. Decreased bone resorption presumably results from impaired acidification, and tartrate-resistant acid phosphatase activity in the serum is actually increased.534-536
URL: https://www.sciencedirect.com/science/article/pii/B9781437703245000298
Quasar Saleem Padiath, Ying-Hui Fu, in Methods in Cell Biology, 2010
Autosomal dominant leukodystrophy (ADLD) is an adult-onset demyelinating disorder that has recently shown to be caused by duplications of the nuclear lamina gene, lamin B1. This chapter attempts to collate and summarize the current knowledge about the disease and the clinical, pathological, and radiological presentations of the different ADLD families described till date. It also provides an overview of the molecular genetics underlying the disease and the mechanisms that may cause the duplication mutation event. ADLD is the first disease that has ever been linked to lamin B1 mutations and it expands the pathological role of the nuclear lamia to include disorders of the brain. The chapter also speculates on the different mechanisms that may link an important and ubiquitous structure like the nuclear lamina with the complex and cell-specific functions of myelin formation and maintenance. Understanding these mechanisms may not only prove helpful in understanding ADLD pathology but can also help in identifying new pathways that may be involved in myelin biology that can have implications for common demyelinating diseases like multiple sclerosis.
URL: https://www.sciencedirect.com/science/article/pii/S0091679X1098014X
Fadil M. Hannan, Rajesh V. Thakker, in Principles of Bone Biology (Fourth Edition), 2020
Autosomal dominant hypocalcemia type 1 (ADH1), which is due to gain-of-function CaSR mutations, is characterized by mild-to-moderate hypocalcemia in association with mild hypomagnesemia; hyperphosphatemia; and serum PTH concentrations that are often detectable and within the lower half of the reference range (Nesbit et al., 2013). Patients with ADH have significantly increased urinary calcium excretion compared with hypoparathyroid patients (Yamamoto et al., 2000), and ∼10% of ADH1 patients have an absolute hypercalciuria (Nesbit et al., 2013). It has been reported that the urinary calcium-to-creatinine (Ca/Cr) ratio may be used to distinguish ADH1 from hypoparathyroidism, with untreated ADH1 patients having a mean Ca/Cr ratio of >3.0 mmol/mmol creatinine, which was similar to the urinary Ca/Cr ratio of unaffected normocalcemic subjects, whereas untreated hypoparathyroid patients had a significantly lower mean Ca/Cr ratio of <0.1 mmol/mmol creatinine (Yamamoto et al., 2000). Some patients with ADH1 may have ectopic calcifications affecting the basal ganglia and/or elevations in bone mineral density (BMD) (Pearce et al., 1996), and some patients with severe gain-of-function CaSR mutations may also develop a Bartter syndrome (referred to as Bartter syndrome type V), which is characterized by renal salt wasting leading to volume depletion, hyperreninemic hyperaldosteronism and hypokalemic alkalosis (Watanabe et al., 2002). Over 70 different CaSR mutations have been identified in ADH1 patients and families (Hannan et al., 2016; Hannan and Thakker, 2013), and structure–function analysis have shown that ADH1-causing gain-of-function mutations cluster within the second peptide loop of the extracellular VFT domain (residues 116–136), which contributes to the dimeric CaSR interface (Geng et al., 2016). A second hotspot for ADH1 mutations is located in a region that encompasses transmembrane helices 6 and 7, and the intervening third extracellular loop of the CaSR (residues 819–837) (Hannan et al., 2016). This transmembrane region may play a role in locking the ligand-unbound receptor in an inactive conformation by forming a network of interactions with other transmembrane helices (Dore et al., 2014).
URL: https://www.sciencedirect.com/science/article/pii/B9780128148419000567
Shibo Tang, ... Yan Luo, in Retina (Fifth Edition), 2013
These conditions are characterized by hereditary peripheral retinal neovascularization with vitreoretinal traction. We discuss here hereditary conditions without primary vitreal degeneration, unaccompanied by systemic clinical manifestations, and incontinentia pigmenti, sickle-cell retinopathy, and other peripheral proliferative retinopathies that have been reviewed previously.151
ADNIV is an apparently rare condition characterized by cataract, cystoid macular edema, peripheral retinal scarring and pigmentation, peripheral arteriolar closure, and neovascularization of the peripheral retina at the ora serrata.152 Young adults are asymptomatic, but have vitreous cell and selective b-wave loss on the ERG. Neovascularization may result in tractional retinal detachment. About half of patients will develop rubeosis or neovascular glaucoma by age 60 or older. The gene was localized to chromosome 11q13.153 Vitreous bands and sheets are not observed and the vitreous was not optically empty, enabling differentiation from classical vitreoretinal degenerations such as Stickler, Wagner, and SVD. The peripheral retinal vessels are initially normal in ADNIV, and dragging of the macular vessels as seen in familial exudative vitreoretinopathy is absent.
Gitter and colleagues described a family with 7 of 15 members affected with early cataract, uveitis, prominence of the vitreous base, lattice degeneration, and severe peripheral retinal neovascularization leading to vitreous hemorrhage and retinal detachment. The syndrome appears similar to ADNIV, but after reviewing photographs of the ADNIV family, the condition was deemed to be distinct.154
URL: https://www.sciencedirect.com/science/article/pii/B9781455707379000412
University College London, London, United Kingdom
The University of British Columbia, Vancouver, Canada
Johns Hopkins School of Medicine, Baltimore, United States
We use cookies to help provide and enhance our service and tailor content and ads. By continuing you agree to the use of cookies.
Copyright © 2021 Elsevier B.V. or its licensors or contributors. ScienceDirect ® is a registered trademark of Elsevier B.V.
ScienceDirect ® is a registered trademark of Elsevier B.V.
Ukraine Bukkake
Phrygian Dominant
Natasha Henstridge Bikini
Celebrity Xxx Scenes
Ovation Celebrity
Autosomal Dominant Inheritance - an overview ...
Autosomal dominant inheritance pattern - Mayo Clinic
Autosomal Dominant Inheritance - an overview ...
In Autosomal Dominant Inheritance Asp Name | Mafia T…
Autosomal dominant inheritance pattern & autosomal ...
Autosomal Dominant - The Definitive Guide | Biology Dictio…
Genetic Inheritance, Autosomal Dominant, X-linked ...
Autosomal dominant inheritance | definition of Auto…
In Autosomal Dominant Inheritance Aspx Cid | .....Sout…
In Autosomal Dominant Inheritance Asp Detid