German Testicular Cancer Study Group

🛑 👉🏻👉🏻👉🏻 INFORMATION AVAILABLE CLICK HERE👈🏻👈🏻👈🏻
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
You have reached the maximum number of saved studies (100).
Please remove one or more studies before adding more.
The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details.
ClinicalTrials.gov Identifier: NCT02229916
Last Update Posted : August 2, 2021
German Testicular Cancer Study Group
Information provided by (Responsible Party):
Dr. med. Christian Rothermundt, Cantonal Hospital of St. Gallen
The majority of testicular cancer patients can be cured with cisplatin-based chemotherapy. Mortality has been reduced even more within the last 15 years due to the stringent application of standard chemotherapy followed by resection of residual disease. This is a positive development considering that testicular cancer usually affects young men. Active surveillance has become an acceptable and widely used strategy in stage I testicular cancer. Thus, it is important to follow these patients in a standardized way and to adhere to a rationale surveillance strategy. There is no international consensus regarding follow-up of testicular cancer patients. Stratification according to risks and patterns of relapse would allow to tailor follow-up schedules, aiming at early identification of relapse without causing unnecessary harm by using excessive radiation in these young long-term survivors. Follow-up procedures should not only aim at detecting relapse, but also long-term side effects from therapy, including hypogonadism, metabolic syndrome, cardiovascular disease and secondary malignancies. The Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS) will comprise consecutive newly diagnosed testicular cancer patients and is the first study to prospectively evaluate the initial indictor of relapse in testicular cancer patients, the frequency and pattern of relapse and document long-term toxicities of the treatment (cardiovascular, gonadal, hearing impairment, renal function and second malignancies) and psychosocial aspects. This cohort study will determine the relevance of each test performed routinely during follow-up. The collected data will have direct implications for the care of patients with testicular cancer and inform future adaptations of follow-up recommendations. The dataset will give information on baseline factors of testicular cancer patients patients, current treatment strategies in Switzerland, Austria and Germany, outcome and late sequelae.
Swiss Austrian German Testicular Cancer Cohort Study - SAG TCCS
Estimated Primary Completion Date :
Diagnostic performance and the clinical impact of a variety of tests, including conventional radiographs, computed tomographies (CT), abdominal ultrasound, serum tumour markers (AFP, beta-HCG and LDH) and clinical signs and symptoms aimed at early detection of relapse after curative therapy with documented complete remission.
Secondary Outcome Measures :
Rate of relapses detected on chest x-ray in seminoma patients [ Time Frame: 8 years ]
Rate of false positive abnormalities on CT scan [ Time Frame: 8 years ]
Rate of false positive tumour marker elevations not due to seminomatous or non-seminomatous germ cell tumour relapses but due to other reasons [ Time Frame: 8 years ]
Patient characteristics at baseline and at the time-point of relapse detection. [ Time Frame: 8 years ]
Rate of stage I seminoma and non-seminoma patients undergoing active surveillance. [ Time Frame: 8 years ]
Overview of treatment and follow-up strategies in germ cell cancer patients in Switzerland, Austria and Germany [ Time Frame: 8 years ]
Treatment sequelae following testicular cancer treatment in terms of organ function, cardiovascular risk factors, sexual health and socioeconomic aspects. [ Time Frame: 8 years ]
Rate of intermediate and poor-prognosis disease at relapse. [ Time Frame: 8 years ]
Rate of offspring spontaneously conceived after testicular cancer treatment. [ Time Frame: 8 years ]
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
18 Years and older (Adult, Older Adult)
Consecutive patients with testicular cancer of any type and any stage and any age (incident cases).
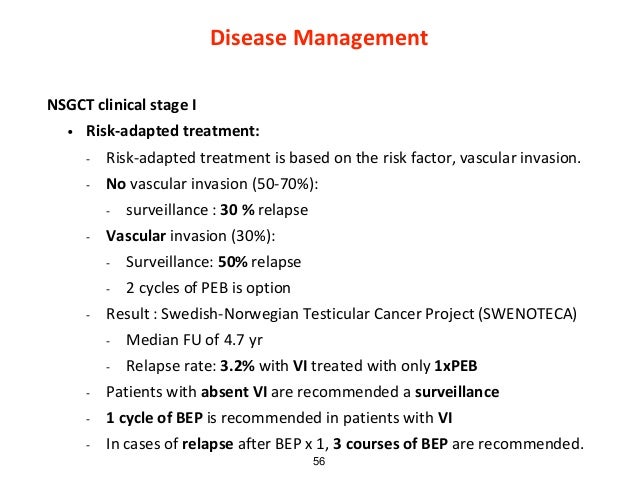
Testicular cancers are generally classified as seminomatous (seminoma) and nonseminomatous germ cell tumours (nonseminoma) of the testis. Mixed germ cell tumours belong to the group of non-seminomas. The stage of disease and the choice of treatment (active surveillance vs. chemotherapy vs. radiotherapy) define the risk of relapse, the pattern of relapse and the long-term toxicities. Staging in testicular cancer is performed according to the American Joint Committee on Cancer (AJCC) TNM staging system for testis cancer [46]. Metastatic testicular cancers are classified according to the International Germ Cell Cancer Collaborative Group (IGCCCG) risk groups [47].
To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor.
Please refer to this study by its ClinicalTrials.gov identifier (NCT number): NCT02229916
Kantonsspital St.Gallen; Onkologie/Haematologie
Contact: Christian Rothermundt, Dr. med. +41 71 494 11 11 christian.rothermundt@kssg.ch
Principal Investigator: Christian Rothermundt, Dr. med.
German Testicular Cancer Study Group
Dr. med. Christian Rothermundt, PD Dr. med., Cantonal Hospital of St. Gallen
September 3, 2014 Key Record Dates
Keywords provided by Dr. med. Christian Rothermundt, Cantonal Hospital of St. Gallen:
Testicular Neoplasms
Testicular Cancer
Cohort Study
Testicular Neoplasms
Neoplasms
Endocrine Gland Neoplasms
Neoplasms by Site
Genital Neoplasms, Male
Urogenital Neoplasms
Endocrine System Diseases
Testicular Diseases
Gonadal Disorders
Format:
Summary (text)
PubMed
PMID
Abstract (text)
CSV
Subject: 1 selected item: 18006893 - PubMed
Format:
Summary
Summary (text)
Abstract
Abstract (text)
Create a new collection
Add to an existing collection
Name must be less than 100 characters
Unable to load your collection due to an error
Please try again
Unable to load your delegates due to an error
Please try again
Would you like email updates of new search results?
Saved Search Alert Radio Buttons
Yes
No
Which day?
The first Sunday
The first Monday
The first Tuesday
The first Wednesday
The first Thursday
The first Friday
The first Saturday
The first day
The first weekday
Report format:
Summary
Summary (text)
Abstract
Abstract (text)
PubMed
Send at most:
1 item
5 items
10 items
20 items
50 items
100 items
200 items
Send even when there aren't any new results
Number of items displayed:
5
10
15
20
50
100
Affiliation
1 Department of Oncology/Hematology, University Medical Centre Eppendorf, Hamburg, Germany.
C Bokemeyer et al. Ann Oncol. 2008 Mar.
Affiliation
1 Department of Oncology/Hematology, University Medical Centre Eppendorf, Hamburg, Germany.
Background: The aim of this study is to determine feasibility and efficacy of the combination regimen gemcitabine, oxaliplatin, and paclitaxel (GOP) in patients with cisplatin-refractory or multiply relapsed germ-cell tumors.
Patients and methods: From April 2003 to October 2006, 41 patients refractory to cisplatin-based chemotherapy or with relapse after high-dose chemotherapy (HDCT) plus stem-cell support (peripheral blood stem-cell transplantation: PBSCT) received 800 mg/m2 gemcitabine, 80 mg/m2 paclitaxel (Taxol), both on days 1 + 8, and oxaliplatin 130 mg/m2 on day 1 of a 3-week cycle for a minimum of two cycles. Primary end point was response rate. Patients were pretreated with a median of two lines of platin-based chemotherapy (range, 1-3), and 78% had relapsed after HDCT/PBSCT.
Results: Seventy-three percent of patients had relapsed within 3 months after the last cisplatin-based chemotherapy. Five percent of the patients achieved a complete response, and 34% and 12% a marker-negative and marker-positive partial response, respectively (overall response rate 51%). After a median follow-up of 5 months (range, 0-20), 15% of the patients remain in complete remission after GOP chemotherapy +/- residual tumor resection with a median response duration of 8 months (1 to 17+). Main toxicity was leucocytopenia grade 3/4 in 15%, anemia in 7%, and thrombocytopenia in 49% of the patients.
Conclusion: Combination chemotherapy with GOP is feasible and effective with acceptable toxicity in patients with treatment-refractory germ-cell tumors.
Seidel C, Oechsle K, Lorch A, Dieing A, Hentrich M, Hornig M, Grünwald V, Cathomas R, Meiler J, de Wit M, Bokemeyer C. Seidel C, et al. Urol Oncol. 2016 Apr;34(4):167.e21-8. doi: 10.1016/j.urolonc.2015.11.007. Epub 2015 Dec 11. Urol Oncol. 2016. PMID: 26699830
Kollmannsberger C, Beyer J, Liersch R, Schoeffski P, Metzner B, Hartmann JT, Rick O, Stengele K, Hohloch K, Spott C, Kanz L, Bokemeyer C. Kollmannsberger C, et al. J Clin Oncol. 2004 Jan 1;22(1):108-14. doi: 10.1200/JCO.2004.06.068. J Clin Oncol. 2004. PMID: 14701772 Clinical Trial.
Oechsle K, Kollmannsberger C, Honecker F, Mayer F, Waller CF, Hartmann JT, Boehlke I, Bokemeyer C; German Testicular Cancer Study Group. Oechsle K, et al. Eur Urol. 2011 Oct;60(4):850-5. doi: 10.1016/j.eururo.2011.06.019. Epub 2011 Jun 24. Eur Urol. 2011. PMID: 21704446
Farmakis D, Pectasides M, Pectasides D. Farmakis D, et al. Eur Urol. 2005 Sep;48(3):400-7. doi: 10.1016/j.eururo.2005.04.024. Eur Urol. 2005. PMID: 15964136 Review.
Oing C, Alsdorf WH, von Amsberg G, Oechsle K, Bokemeyer C. Oing C, et al. World J Urol. 2017 Aug;35(8):1167-1175. doi: 10.1007/s00345-016-1898-z. Epub 2016 Jul 23. World J Urol. 2017. PMID: 27449639 Review.
Abughanimeh O, Teply BA. Abughanimeh O, et al. Curr Oncol Rep. 2021 Jul 16;23(9):101. doi: 10.1007/s11912-021-01093-z. Curr Oncol Rep. 2021. PMID: 34269906 Review.
Oing C, Hentrich M, Lorch A, Gläser D, Rumpold H, Ochsenreither S, Richter S, Dieing A, Zschäbitz S, Pereira RR, Bokemeyer C, Seidel C. Oing C, et al. J Cancer Res Clin Oncol. 2020 Feb;146(2):449-455. doi: 10.1007/s00432-019-03071-2. Epub 2019 Dec 14. J Cancer Res Clin Oncol. 2020. PMID: 31838576
Bremmer F, Bohnenberger H, Küffer S, Oellerich T, Serve H, Urlaub H, Strauss A, Maatoug Y, Behnes CL, Oing C, Radzun HJ, Ströbel P, Balabanov S, Honecker F. Bremmer F, et al. Dis Markers. 2019 Sep 3;2019:8298524. doi: 10.1155/2019/8298524. eCollection 2019. Dis Markers. 2019. PMID: 31565104 Free PMC article.
Nair PR. Nair PR. Polymers (Basel). 2019 Apr 5;11(4):630. doi: 10.3390/polym11040630. Polymers (Basel). 2019. PMID: 30959799 Free PMC article. Review.
Bremmer F. Bremmer F. Pathologe. 2018 Dec;39(Suppl 2):215-220. doi: 10.1007/s00292-018-0493-z. Pathologe. 2018. PMID: 30206653 Review. German.
Girls Seduced Porn
Extreme Bbc Titans Orgy Full Torrent
Futa On Female Comics
Retro Taboo Kay Parker Xxx
Mom Son Stories
Swiss Austrian German Testicular Cancer Cohort Study - SAG ...
Combination chemotherapy with gemcitabine, oxaliplatin ...
Treatment of patients with cisplatin-refractory testicular ...
GTCSG - German Testicular Cancer Study Group
Swiss Austrian German Testicular Cancer Cohort Study - SAG ...
Risk factors for relapse in stage I non-seminomatous germ ...
Randomized Phase III Trial Comparing Retroperitoneal Lymph …
Ergebnisse der randomisierten Phase-III-Studie der „German ...
ASCO 2010 Highlights: Testicular Cancer
Ergebnisse der randomisierten Phase-III-Studie der „German ...
German Testicular Cancer Study Group