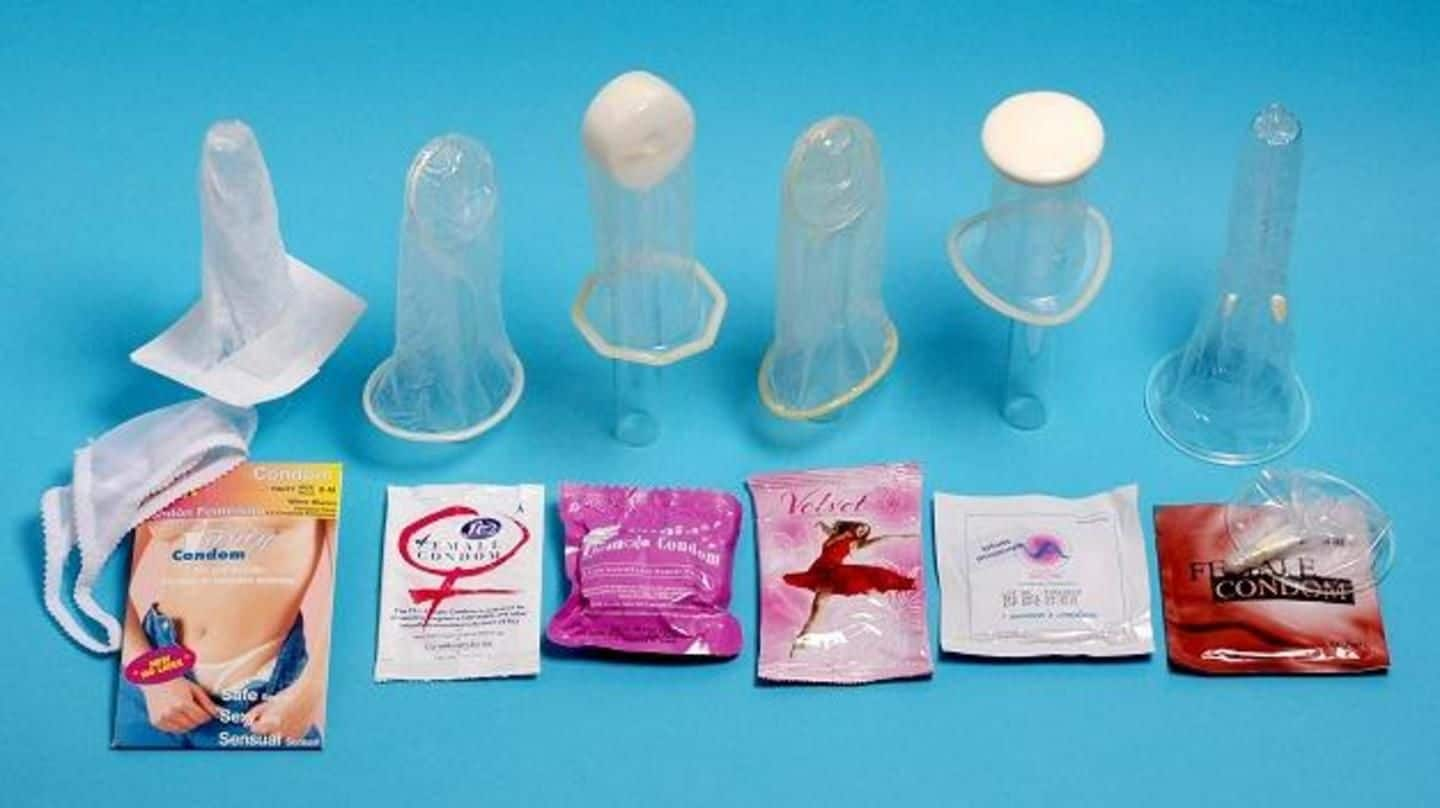
Female Condom

🛑 👉🏻👉🏻👉🏻 INFORMATION AVAILABLE CLICK HERE👈🏻👈🏻👈🏻
Don't delay your care at Mayo Clinic
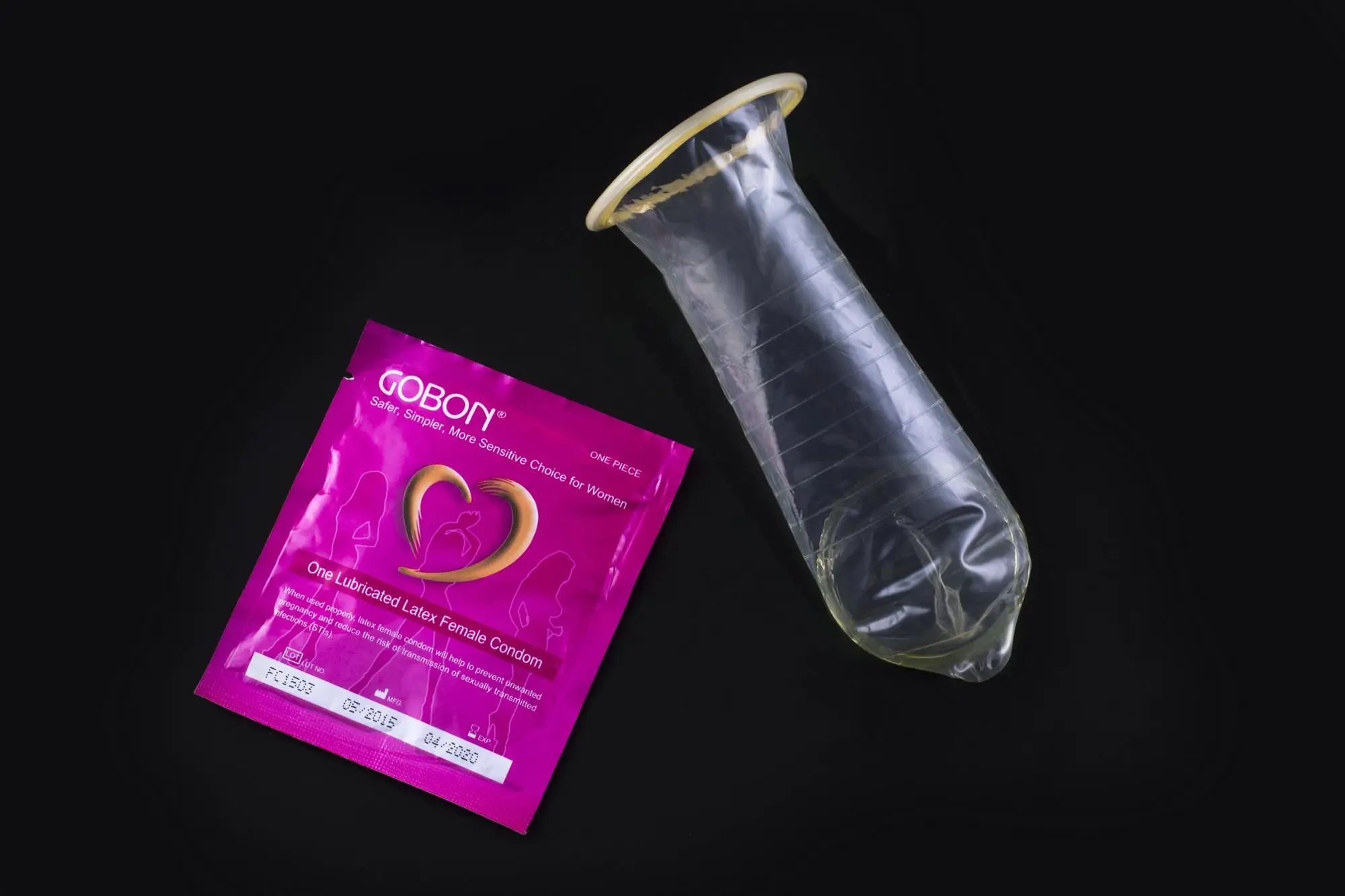
A female condom is a soft, loosefitting pouch that's inserted into the vagina before sex to prevent pregnancy and sexually transmitted infections.
The female condom — also called an internal condom — is a birth control (contraceptive) device that acts as a barrier to keep sperm from entering the uterus. It protects against pregnancy and sexually transmitted infections (STIs).
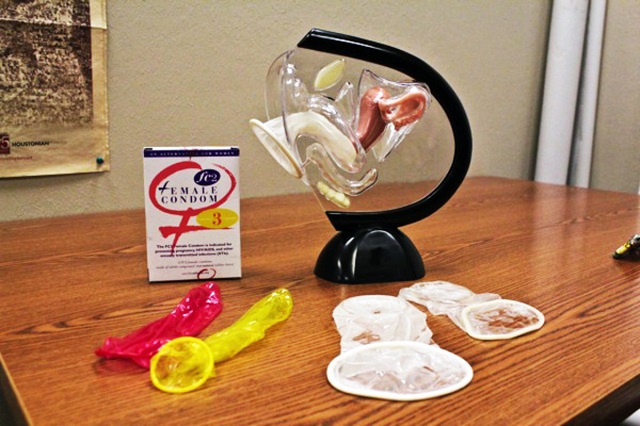
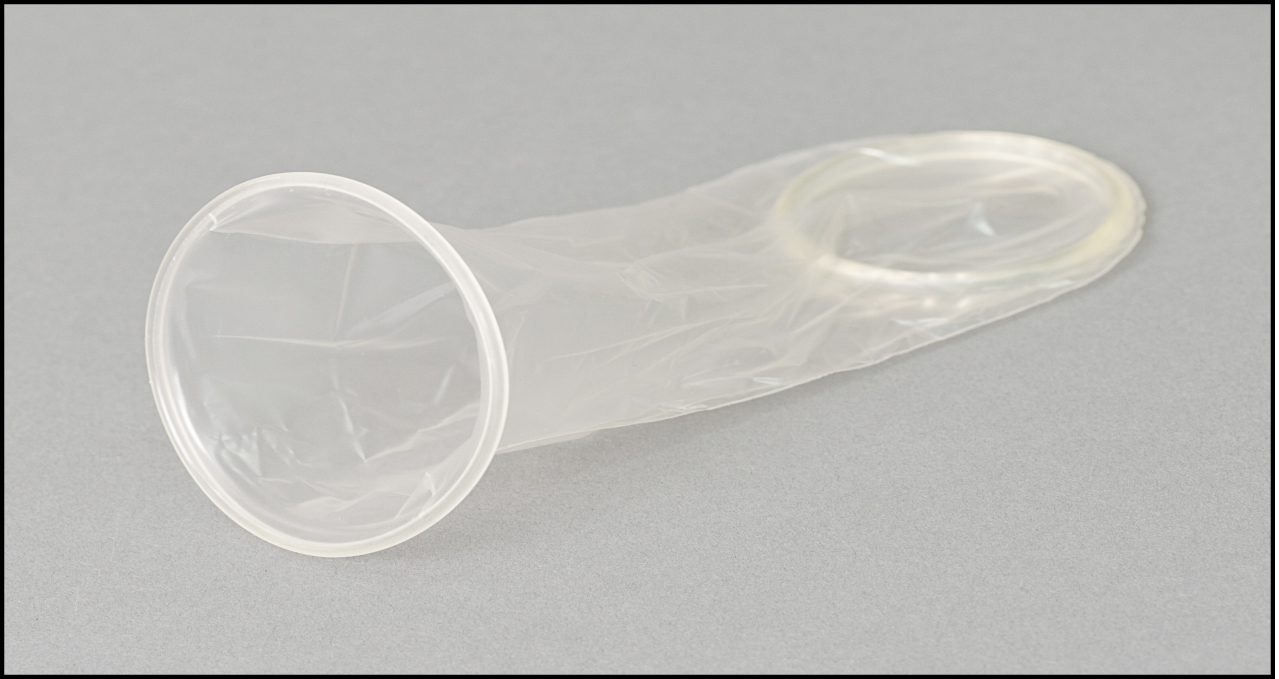
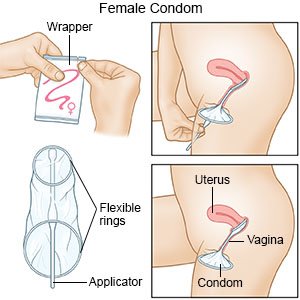
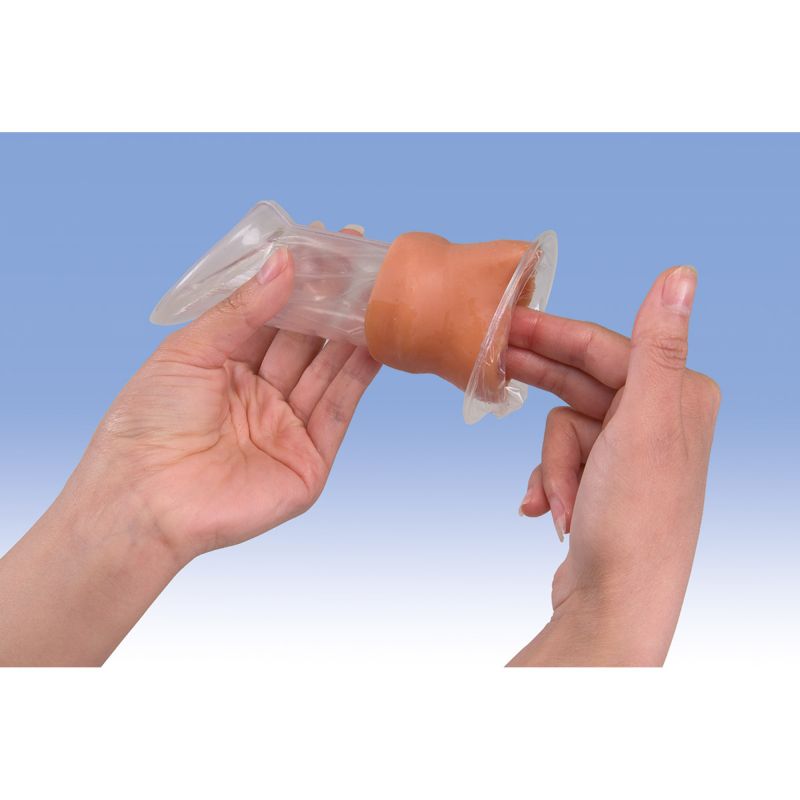
The female condom is a soft, loosefitting pouch with a ring on each end. One ring is inserted into the vagina to hold the female condom in place. The ring at the open end of the condom remains outside the vagina. The outer ring helps keep the condom in place and is also used for removal. The female condom can be used during anal sex, too.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
Only two female condoms — the FC1 female condom and its replacement, the FC2 female condom — have Food and Drug Administration approval in the U.S. The FC1 female condom, which is made of plastic (polyurethane), is no longer being produced. The FC2 female condom is made of synthetic latex — safe for those with allergies to natural rubber latex — and is pre-lubricated with a silicone-based lubricant.
The female condom helps prevent pregnancy. You may also use a female condom to protect against sexually transmitted infections (STIs) during anal sex. Among various benefits, the female condom:
According to the FC2 website, the FC2 is reimbursable if you have insurance and a prescription from a doctor. Health care organizations that offer web-based (virtual) visits also may allow you to obtain a prescription to send to a pharmacy. If you don't have insurance, you can directly purchase the female condom from the website. FC2 may also be available through universities and community health-based organizations such as Planned Parenthood.
Unlike latex — the material used to make most male condoms — female condoms are made of polyurethane and synthetic latex, which is safe for people who are allergic to natural rubber latex. Female condoms aren't affected by dampness or changes in temperature. In addition, some women find that the female condom's external ring stimulates the clitoris.
The female condom isn't appropriate for everyone, however. You may want to consider another type of birth control if you:
Up to 21 out of 100 women will become pregnant in a year of typical use of female condoms — possibly because they don't use condoms every time they have sex.
The female condom has a higher failure rate than the male condom. Condom failure means it's possible to contract sexually transmitted infections or become pregnant. The female condom may not protect you if:
The female condom may also cause discomfort during insertion, a burning sensation, itching or a rash.
Before using a female condom, read the instructions carefully. If the condom is past its expiration date or you notice any signs of damage — such as small tears or holes — discard the condom and choose another.
Practice inserting the female condom before the first time you use it for sex. In addition, pay close attention when you first use the female condom to make sure it stays in place during sex. Never reuse a female condom.
Don't use a female condom at the same time as a male condom. They can stick together, which might cause one or both condoms to break. The female condom isn't currently FDA-approved for anal sex.
To use a female condom, one ring is inserted into the vagina before sex to hold the condom in place. The ring at the open end of the female condom remains outside the vagina.
Insert the female condom. Squeeze the ring at the closed end of the pouch with your middle finger and thumb and insert it into your vagina like a tampon. Place your index finger inside the condom and push the ring up as far as it will go.
Don't allow the condom to twist. Make sure the outer ring remains outside the vagina, extending about 1 inch (or about 2.5 centimeters) beyond the labia. You can place the female condom inside your vagina up to eight hours before sex.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
Hoke TH, et al. Female condoms. http://www.uptodate.com/contents/search. Accessed Nov. 3, 2017.
Hatcher RA, et al., eds. Managing contraception 2017-2018. Tiger, Ga.: Bridging the Gap Foundation. Accessed Nov. 3, 2017.
Hatcher RA, et al. Vaginal barriers and spermicides. In: Contraceptive Technology. 20th ed. New York, N.Y.: Ardent Media; 2011:391.
The right way to use a female condom. Centers for Disease Control and Prevention. https://www.cdc.gov/condomeffectiveness/Female-condom-use.html. Accessed Nov. 14, 2017.
Lobo RA, et al. Family planning. In: Comprehensive Gynecology. 7th ed. Philadelphia, Pa.: Elsevier; 2017. https://www.clinicalkey.com. Accessed Nov. 14, 2017.
Birth control: Medicines to help you. U.S. Food and Drug Administration. https://www.fda.gov/forconsumers/byaudience/forwomen/freepublications/ucm313215.htm#BarrierMethods. Accessed Nov. 14, 2017.
Laughlin-Tommaso SK (expert opinion). Mayo Clinic, Rochester, Minn. Dec. 11, 2017.
Latex allergy. American College of Allergy, Asthma and Immunology. http://acaai.org/allergies/types/skin-allergies/latex-allergy. Accessed Dec. 28, 2017.
Groch B (expert opinion). Veru Inc. Miami, Fla. Oct. 12, 2017.
How to find. FC2. https://fc2.us.com/patient. Accessed Jan. 25, 2018.
Product classification: Single-use internal condom. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=4062. Accessed Feb. 5, 2020.
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
© 1998-2021 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.
A female condom (also known as a femidom or internal condom) is a barrier device that is used during sexual intercourse as a barrier contraceptive to reduce the probability of pregnancy or a sexually transmitted infection (STI). Meant as an alternative to the condom for males, it was invented by Danish MD Lasse Hessel and designed to be worn internally by the female partner during vaginal sex to prevent exposure to ejaculated semen or other body fluids. His invention was launched in Europe in 1990 and approved by the FDA for sale in the USA in 1993.[2] Its protection against STIs is inferior to that of male condoms.[3] Internal condoms can be used by the receptive partner during anal sex.[4][5]
To avoid risk of incorrect use, read the instructions carefully prior to use.
No external drugs or clinic visits required
The female condom is a thin, soft, loose-fitting sheath with a flexible ring/frame or ring/foam disc at the closed end. They typically come in various sizes. For most vaginas, a moderately sized condom is adequate; women who have recently given birth should try a large size first. The inner ring or foam disc at the closed end of the sheath is used to insert the condom inside the vagina and to hold it in place during intercourse. The rolled outer ring or poly frame at the open end of the sheath remains outside the vagina and covers part of the external genitalia.
The female condom was developed in the late 20th century (male condoms have been used for centuries). A primary motive for its creation is the well-documented refusal of some men to use a condom because of loss of sensation and the resulting impact on the hardness of the man's erection, and secondarily by its implication that the male could transmit an STI.[6][7]
The FC1 female condom was first made from polyurethane. The second generation female condom is called the FC2 and is made from synthetic nitrile[8] (this material change was announced in September 2005,[9] and full transition of the product line to FC2 was done by October 2009[10]). The newer nitrile condoms are less likely to make potentially distracting crinkling noises. FC2 was developed to take the place of FC1, providing the same safety and efficacy during use, but at a significantly lower cost.[9] FC2 is manufactured by The Female Health Company. The World Health Organization (WHO) has cleared FC2 for purchase by U.N. agencies and the United Nations Population Fund has incorporated the female condom into national programming.[11] They are sold under many brand names, including Reality, Femidom, Dominique, Femy, Myfemy, Protectiv and Care.
A recent version of the female condom is made from natural latex, the same material used in male condoms. This condom does not make the noises some experience with plastic condoms and fits snugly against the female anatomy. This type of female condom is manufactured by HLL Lifecare Ltd, India and IXu LLC of USA. It is sold under the brand name VA w.o.w Condom Feminine. One more clinical trial is required before it can be considered for Food and Drug Administration (FDA) approval in the United States.[12]
The global health nonprofit PATH has also developed a female condom tailored for use in developing countries. The Woman's Condom is manufactured by Shanghai Dahua Medical Apparatus in China and is in early introduction.[13]
The FC2 female condom is a nitrile sheath or pouch 17 cm (6.7 in) in length. At each end there is a flexible ring. At the closed end of the sheath, the flexible ring is inserted into the vagina to hold the female condom in place. The other end of the sheath stays outside the vulva at the entrance to the vagina. This ring acts as a guide during penetration and stops the sheath from shifting during intercourse. There is a silicone-based lubricant on the inside of the condom, but additional lubrication can be used. The condom does not contain spermicide.
The VA w.o.w. Condom Feminine is manufactured by HLL Lifecare Ltd and IXu LLC. This latex condom has a pouch attached to its rounded triangular opening and a sponge to secure it inside a woman's vagina. It is available through the distributor IXu LLC for the private and public sectors in several regions, including the EU, South Africa, Brazil, and India. The VA w.o.w. received the CE mark, a certification that it meets European Union consumer health requirements. It is also under review by the WHO.[14]
The Woman's Condom, developed by PATH, through a user-centered design process, is a new female contraceptive designed for improved acceptability, ease of use, and good sensation. The Woman's Condom is a polyurethane pouch that is partially enclosed in a capsule to aid insertion. The capsule dissolves quickly after insertion in the vagina, which releases the pouch. The condom is then held stable in the woman by foam pads. The Woman's Condom is packaged dry and comes with a small sachet of water-based lubricant to be applied at point of use. PATH licensed manufacturing and distribution of the Woman's Condom to the Shanghai Dahua Medical Apparatus Company in 2008. Dahua has received the South Africa Bureau of Standards (SABS) certification marking (2013), Shanghai Food and Drug Administration Approval (2011), and the CE Mark approval (2010) for the Woman's Condom, which allows for marketing and distribution of the product in South Africa, China and Europe, respectively. The Woman's Condom is currently under review by the WHO/United Nations Population Fund Technical Review Committee; the Committee's approval could lead to bulk public-sector purchase by United Nations agencies.[15]
The Natural Sensation Panty Condom is distributed in the US exclusively by the ACME Condom Company. It is manufactured by Natural Sensation Compañia Ltda. (NS) based in Bogotá, Colombia. The product is made of a polyethylene resin, which is stronger and thinner than latex. Unlike latex, polyethylene is anti-allergenic, ultra sensitive, transparent and odorless. Natural Sensation's condoms are lubricated and may be used with either oil- or water-based lubricants.
The Female Panty Condom is manufactured by Silk Parasol. It is made of biodegradable latex. It has not yet been approved by the FDA and is currently undergoing clinical trials.
The Phoenurse is made of a dumbbell-shaped polyurethane sheath and comes with an insertion tool, a water- or silicone-based lubricant, sanitary towels, and disposal bags. It is manufactured by the Tianjin Condombao Medical Polyurethane Tech. Co. Ltd. and is approved for sale in the European Economic Area. The Phoenurse female condom is also available in Brazil, Sri Lanka, China, Kenya, and Mexico. It has not been approved by the FDA.
The Cupid's Female Condom is made of natural latex rubber and manufactured in India by Cupid Ltd. It is approved for distribution in Europe and was prequalified for distribution by WHO in 2012. It is currently undergoing clinical trials to gain approval by the FDA.
The ORIGAMI Female Condom (OFC) is fabricated in molded silicone for anatomical conformity. It was validated as 100% biocompatible and non-allergenic in independent pre-clinical lab testing. The condom is not yet approved for sale and must be reviewed by the WHO, the C-Mark (EU), and the FDA to meet regulatory safety requirements. The OFC is in clinical trials in San Francisco, California, in collaboration with the Women's Global Health Imperative at RTI, International. Large-scale clinical trials were to follow in 2014, to evaluate its performance and safety. It had been expected to reach the market in late 2015, pending regulatory pre-market approvals. As of January 2017, no results were available from initial feasibility studies.[16] A pending lawsuit involving allegations of embezzlement means that the Origami is, as of February 2017, suspended indefinitely from reaching the market.[17]
The per unit price of female condoms is higher than male condoms but there is some evidence to suggest that polyurethane female condoms can be washed, disinfected, and reused.
Re-using the polyurethane female condom is not considered as safe as using a new one; however, the WHO says,[18]
Batches of new, unused female condoms were subjected to seven cycles of disinfection, washing, drying and re-lubrication, reflecting the steps and procedures in the draft protocol, but at considerably higher concentrations of bleach and for longer durations. All female condom batches met the manufacturing quality assessment specifications for structural integrity after the test cycles. … Disinfection, washing, drying, re-lubrication and reuse of the device were not associated with penile discharge, symptomatic vaginal irritation or adverse colposcopic findings in study volunteers.
A presentation at the 1998 International AIDS conference concluded that "washing, drying and re-lubricating the female condom up to ten times does not significantly alter the structural integrity of the device. Further microbiological and virological tests are required before re-use of the female condom can be recommended."[19]
Research suggests that the FC2 female condoms are a cost-effective method of HIV prevention even at low levels of use. The data shows that the cost-effectiveness would increase significantly at higher levels of use. A study conducted in 2005 by David Holtgrave, Chair of the Department of Health, Behavior and Society at Johns Hopkins University's Bloomberg School of Public Health, examined the projected public health impact that the FC2 female condom would have at different levels of use in two developing countries: South Africa and Brazil. The study concluded that FC2 use would generate significant cost savings at all levels of implementation by preventing thousands of HIV infections and saving millions of dollars in health care costs.[20] There is some evidence to suggest that the effectiveness of female condoms in preventing transmission of HIV may be similar to that of male condoms.[21]
As with all barrier contraceptives, water or silicone-based lubricants are safe to use with any female condom. Oil should not be used with a female condom made of latex.
The FC2 Female Condom comes pre-lubricated with a non-spermicidal, silicone based lubricant. The FC2 is made of nitrile so oil-based (or water-based) lubricants can be added on the inside and outside of the FC2 Female Condom or on the penis.
When used correctly, the female condom has a 5% failure rate. Inconsistent or incorrect usage has been shown to result in a 21% failure rate.[22]
Some benefits of female condoms over other methods of birth control include:[23]
Some disadvantages to the female condom include:[24]
FC2 Female Condom gives women control and choice over their own sexual health; women can protect themselves when their partner does not want to use a male condom; female condoms may provide enhanced sensation for men as compared to male condoms;[25][26] FC2 is hypo allergenic and is safe to use with people who are allergic to rubber latex; FC2 may be inserted hours before intercourse; female condoms are not dependent on the penis being erect for insertion and does not require immediate withdrawal after ejaculation; FC2 is not tight or constricting; FC2 is highly lubricated and the material warms to body temperature.[27]
The external genitals of the wearer and the base of the penis of the inserting partner may be better protected (from skin-to-skin transmitted STDs such as herpes and HPV) than when the male condom is used; however see studies below.[citation needed]
Sales of female condoms have been low in developed countries, though developing countries are increasingly using them to complement already existing family planning and HIV/AIDS programming.[28] Probable causes for poor sales are that inserting the female condom is a skill that has to be learned and that female condoms can be significantly more expensive than male condoms (upwards of 2 or 3 times the cost). Also, reported "rustling" sounds from the original version of the female condom during intercourse turn off some potential users, as does the visibility of the outer ring which remains outside the vagina.[29] Regulatory issues have also limited interest in manufacturing female condoms. In the United States, the FDA historically categorized female condoms as Class III medical devices, a category with more stringent requirements than Class II, which includes external condoms. Following proposals to reclassify female condoms,[30] the FDA announced in 2018 that single-use female condoms would now be known as single-use internal condoms, and moved to Class II.[31]
In November 2005, the World YWCA called on national health ministries and intern
Tits Sara
Black Dick Xvideos
Big Women Bikini
Vicky Love Premium Bukkake Gangbang
Hot Celebrity Sex
Female condom - Mayo Clinic
Female condom - Wikipedia
Female Condom

/cdn.vox-cdn.com/uploads/chorus_asset/file/10469861/CONDOM_VOX.jpg)