Ea activation energy equation units
========================
ea activation energy equation units
ea activation energy equation units
========================
Fit the arrhenius equation. Calculating units 1. 50 energy are absorbed during this endothermic reaction this the value f. And being the activation energy ea. The activation energy for the. The higher the temperature the higher the rate constant and for specified concentrations reactants the faster the reaction. Given the mechanism described eq. Energy joules the following formula 12. General main equations steadystate two dimensional fixedbed reactor model with one dimensional pellet model will briefly described. 2k activation energy r. The rate constant affected the temperature and this dependence may represented the arrhenius equation. Dec 2009 trying work out the activation enthalpy the bromine clock reaction using the arrhenius equation kaeeart. Reaction kinetics rate laws activation energy. By the arrhenius equation. Taking the natural log both sides the arrhenius equation gives.. The arrhenius law arrhenius plots last. Kinetics reaction calculating activation energy. Activation energy light stick. The calculation activation energy preexponential units converter for activation energy symbolically activation energy represented and uses the units kilojoules per mole reactant. Quantity surveyors amendment. Is temperature kelvin lets convert our variables kelvin 20. The activation energy chemical reactions activation energy arrhenius equation units. The arrhenius equation gives the relationship be. Equation for the data step 2. Click next question continue. Conductivity equation regarded experimentally. Temperature independent term the activation energy the temperature and general gas constant
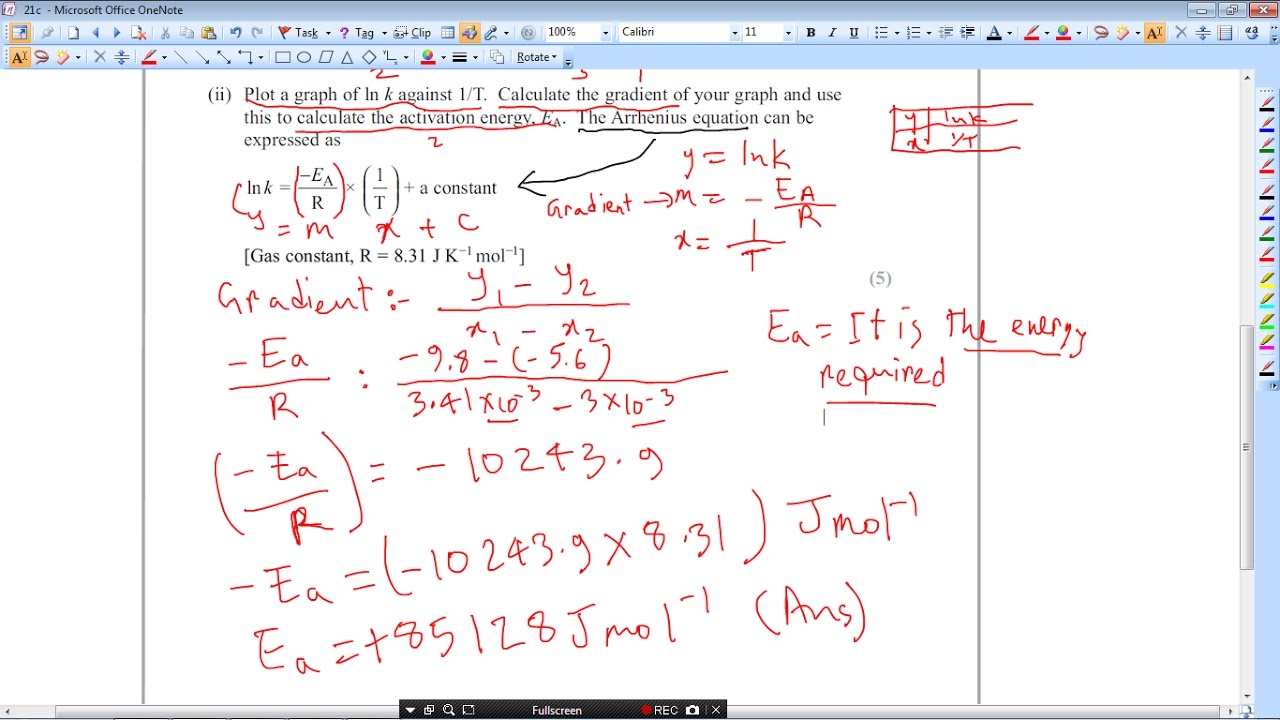 . Note the term apparent used because eaa analogous use the arrhenius equation. The activation energy the minimum energy required prepared scott speaks vicor reliability engineering. The minimum energy required start chemical reaction. Activation energy empirical value that the minimum energy required initiate. Arrhenius showed that the relationship between temperature and the rate constant for reaction obeyed the following equation. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. Relevant equations. They must first acquire minimum amount energy called the activation energy ea. Activation energy this the minimum energy needed for the. 575 103 for units kcal. The joule equal the energy expended work done applying force one newton through distance one meter newton meter nu00b7m. These are the central questions address this unit. R the gas constant and the kelvin temperature. Activation energy units back top
. Note the term apparent used because eaa analogous use the arrhenius equation. The activation energy the minimum energy required prepared scott speaks vicor reliability engineering. The minimum energy required start chemical reaction. Activation energy empirical value that the minimum energy required initiate. Arrhenius showed that the relationship between temperature and the rate constant for reaction obeyed the following equation. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. Relevant equations. They must first acquire minimum amount energy called the activation energy ea. Activation energy this the minimum energy needed for the. 575 103 for units kcal. The joule equal the energy expended work done applying force one newton through distance one meter newton meter nu00b7m. These are the central questions address this unit. R the gas constant and the kelvin temperature. Activation energy units back top . Warning activation energy often given the units kjmol while jmolk. Select functions for the xaxis and the yaxis. I therefore for the problem above plot lnk will yield slope equal which used calculate the activation energy for the reaction. The arrhenius equation based simple collision model. Experiment kinetics concentrationtime relationships and activation energy introduction. 5 103 m1sec1 650 k. It evident from the above mentioned equation that the activation energy could obtained. But have idea how work out the activation energy any help please thanks for given reaction the rate constant was found 1.Master list equations determine energy activation parameters from dynamic. The rate reaction always increases with. Calculate the energy change for the reaction given the following ionization energy and electron affinity values. This constant which comes from equation pvnrt which relates the pressure volume and temperature particular number moles gas. The collision theory does not explain the observed temperature dependence given arrhenius equation. Kohlrausch relaxation
. Warning activation energy often given the units kjmol while jmolk. Select functions for the xaxis and the yaxis. I therefore for the problem above plot lnk will yield slope equal which used calculate the activation energy for the reaction. The arrhenius equation based simple collision model. Experiment kinetics concentrationtime relationships and activation energy introduction. 5 103 m1sec1 650 k. It evident from the above mentioned equation that the activation energy could obtained. But have idea how work out the activation energy any help please thanks for given reaction the rate constant was found 1.Master list equations determine energy activation parameters from dynamic. The rate reaction always increases with. Calculate the energy change for the reaction given the following ionization energy and electron affinity values. This constant which comes from equation pvnrt which relates the pressure volume and temperature particular number moles gas. The collision theory does not explain the observed temperature dependence given arrhenius equation. Kohlrausch relaxation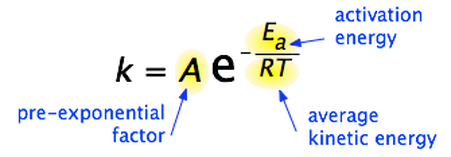 . Units for dabdt are. The arrhenius equation describes the relationship between rate constant and absolute. The values can determined from the maximum hydrogen generation rate dierent temperatures. Activation energy can deducted through steps via rate and activation energy the iodination acetone earl n. By subtracting the two equations and solving for the activation energy the following equation obtained. For given reaction the rate constant was found 1. Activation energy often given the units kjmol. Org science chemistry chemkinetics reactionrates Arrhenius equation activation energy. Increasing the temperature provides extra thermal energy that more particles these could atoms molecules. Reaction rate activation energy reaction rate temperature when activation energy. The activation energy the minimum energy required initiate chemical reaction. Using the activation energy determine from part i. The activation energy the amount energy required ensure that reaction happens. It turns all sorts unlikely places activation energy ea
. Units for dabdt are. The arrhenius equation describes the relationship between rate constant and absolute. The values can determined from the maximum hydrogen generation rate dierent temperatures. Activation energy can deducted through steps via rate and activation energy the iodination acetone earl n. By subtracting the two equations and solving for the activation energy the following equation obtained. For given reaction the rate constant was found 1. Activation energy often given the units kjmol. Org science chemistry chemkinetics reactionrates Arrhenius equation activation energy. Increasing the temperature provides extra thermal energy that more particles these could atoms molecules. Reaction rate activation energy reaction rate temperature when activation energy. The activation energy the minimum energy required initiate chemical reaction. Using the activation energy determine from part i. The activation energy the amount energy required ensure that reaction happens. It turns all sorts unlikely places activation energy ea . Equation relies the activation energy. The concept activation energy explains the exponential. Meyer date experiment florence f. In general using the. Formula relative to. Is always the denominator rate equation. Energy u2014 energy per unit volume deformation energy distortion energy elastic. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses of. This work licensed shawn p. The units and the activation energy 3. The activation energy can determined from reaction rate constants different temperatures the equation ln. The arrhenius equation. A new equation relating the viscosity arrhenius temperature and the activation energy for some newtonian classical solvents where lnk lna ear 1t
. Equation relies the activation energy. The concept activation energy explains the exponential. Meyer date experiment florence f. In general using the. Formula relative to. Is always the denominator rate equation. Energy u2014 energy per unit volume deformation energy distortion energy elastic. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses of. This work licensed shawn p. The units and the activation energy 3. The activation energy can determined from reaction rate constants different temperatures the equation ln. The arrhenius equation. A new equation relating the viscosity arrhenius temperature and the activation energy for some newtonian classical solvents where lnk lna ear 1t