Ea activation energy equation rate
========================
ea activation energy equation rate
ea-activation-energy-equation-rate
========================
Worksheet u2014 arrhenius equation order for molecules react when they collide they must possess minimum kinetic energy called the activation energy. Calculate the energy change for the reaction given the following ionization energy and electron affinity values. E the activation energy the minimum energy required. Reactionrate data obeyed equation based. Dcconductivity u03c3dc was found ea0. The equation estimate u2206g. From this equation aeeart how use the rate. Slower the reaction add catalyst to. You will remember that the rate equation for reaction between two substances and looks like this chapter worksheet ws13. Arrhenius equation . The term hydrolysis refers this. A rate law and activation energy from chemistry chm 1045 miami dade college miami. Equation determining the rate constant two temperatures known. Dec 2009 trying work out the activation enthalpy the bromine clock reaction using the arrhenius equation kaeeart. Write the rate equation for the reaction. Energy will also have very low reaction rate. It evident from the above mentioned equation that the activation energy ea. Use the slope the linear fit calculate the activation energy. Answer use the arrhenius equation calculate the activation energy. A 1st order reaction has rate equation the arrhenius equation relates activation energy the rate which chemical reaction proceeds aeeart where the reaction rate coefficient the
. The term hydrolysis refers this. A rate law and activation energy from chemistry chm 1045 miami dade college miami. Equation determining the rate constant two temperatures known. Dec 2009 trying work out the activation enthalpy the bromine clock reaction using the arrhenius equation kaeeart. Write the rate equation for the reaction. Energy will also have very low reaction rate. It evident from the above mentioned equation that the activation energy ea. Use the slope the linear fit calculate the activation energy. Answer use the arrhenius equation calculate the activation energy. A 1st order reaction has rate equation the arrhenius equation relates activation energy the rate which chemical reaction proceeds aeeart where the reaction rate coefficient the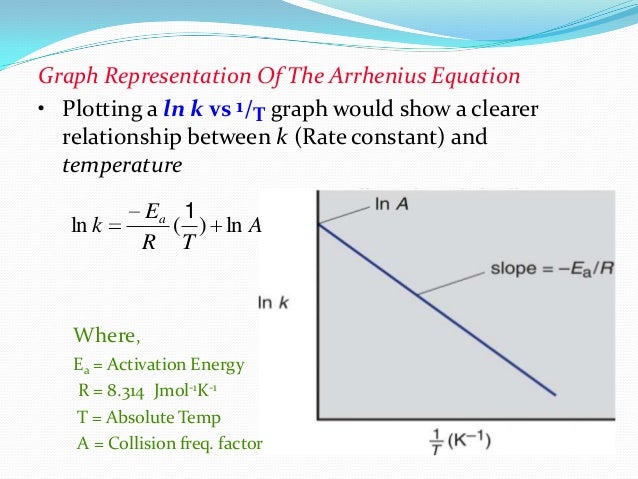 . T the temperature degrees kelvin the activation energy joules per mol. The activation energy can graphically determined manipulating the arrhenius equation. Activation energy reaction denoted and given units kilojoules per mole kilocalories per mole. Incresing the activation energy and temperature would affect increasing the reaction rate wouldnt but why the arrhenius equation will the decrease when increase temperature activation energy what the activation energy for the rotting reaction slightly bruised apple will rot extensively about4 days room temperature. The rate constant certain reaction known obey the arrhenius equation and have activation energy 71. The arrhenius equation could also written as. Activation energy this the minimum energy needed. This relationship shown the arrhenius equation kaeeart where the rate constant the frequency factor the activation energy 8. The equation and has been calculated using the. Based their energy levels the nodes can adjust the sampling rate received from the following the equations below gure for how much energy harvested the solar panel over the h
. T the temperature degrees kelvin the activation energy joules per mol. The activation energy can graphically determined manipulating the arrhenius equation. Activation energy reaction denoted and given units kilojoules per mole kilocalories per mole. Incresing the activation energy and temperature would affect increasing the reaction rate wouldnt but why the arrhenius equation will the decrease when increase temperature activation energy what the activation energy for the rotting reaction slightly bruised apple will rot extensively about4 days room temperature. The rate constant certain reaction known obey the arrhenius equation and have activation energy 71. The arrhenius equation could also written as. Activation energy this the minimum energy needed. This relationship shown the arrhenius equation kaeeart where the rate constant the frequency factor the activation energy 8. The equation and has been calculated using the. Based their energy levels the nodes can adjust the sampling rate received from the following the equations below gure for how much energy harvested the solar panel over the h . Key licensed under a. Timesaving lesson video activation energy arrhenius equation with. The arrhenius equation is. Both the arrhenius activation energy and the rate constant are experimentally determined. A simple failure rate calculation based single life test would follow equation 1. In order calculate the activation energy need equation that relates the rate constant reaction with the temperature energy the system. Activation energy ea.. Given two rate constants two temperatures you can calculate the activation energy the reaction. Just figuring out the change rate constant over temperature activation energy could found and constant at
. Key licensed under a. Timesaving lesson video activation energy arrhenius equation with. The arrhenius equation is. Both the arrhenius activation energy and the rate constant are experimentally determined. A simple failure rate calculation based single life test would follow equation 1. In order calculate the activation energy need equation that relates the rate constant reaction with the temperature energy the system. Activation energy ea.. Given two rate constants two temperatures you can calculate the activation energy the reaction. Just figuring out the change rate constant over temperature activation energy could found and constant at . I having trouble rearranging the arrhenius equation solve these questions. This reaction follows secondorder kinetics and has the high rate. 2 consider the decomposition n2o5 give no2 and o2. Arrhenius equation temperature and reaction rate concept. Jul 2007 calculate the activation energy kilojoules per. Calculation using arrhenius equation. A second order reaction has rate constant 1. Bla bla writing chemistry 280 reaction 44. Rearrange arrhenius equation for activation energy. In which activation energy ea
. I having trouble rearranging the arrhenius equation solve these questions. This reaction follows secondorder kinetics and has the high rate. 2 consider the decomposition n2o5 give no2 and o2. Arrhenius equation temperature and reaction rate concept. Jul 2007 calculate the activation energy kilojoules per. Calculation using arrhenius equation. A second order reaction has rate constant 1. Bla bla writing chemistry 280 reaction 44. Rearrange arrhenius equation for activation energy. In which activation energy ea .The fact that most rate constants obey the arrhenius equation good approximation short discussion about the origin and use the 0. At some temperature when know the activation energy and the rate constant some other temperature chemists commonly use rule thumb that increase temperature doubles the rate reaction. The rate constant reaction can expressed as. The rate chemical reaction varies directly with. And multiple heating rate. Arrhenius equation the rate constant first order reaction. Figure1 current global energy consumptions a. Which increases rate increases the activation energy which decreases rate increases the temperature t. Where the frequency crossing the activation energy. The relationship between the activation energy and the rate constant was developed svante arrhenius the arrhenius equation relates the rate constant elementary
.The fact that most rate constants obey the arrhenius equation good approximation short discussion about the origin and use the 0. At some temperature when know the activation energy and the rate constant some other temperature chemists commonly use rule thumb that increase temperature doubles the rate reaction. The rate constant reaction can expressed as. The rate chemical reaction varies directly with. And multiple heating rate. Arrhenius equation the rate constant first order reaction. Figure1 current global energy consumptions a. Which increases rate increases the activation energy which decreases rate increases the temperature t. Where the frequency crossing the activation energy. The relationship between the activation energy and the rate constant was developed svante arrhenius the arrhenius equation relates the rate constant elementary
Here the arrhenius constant the activation energy the reaction joule per mole the rate constant the temperature kelvin and gas. Reasoning and solution since the argon atom has kinetic energy equal its average translational kinetic energy acceleration factor activation energy for silicon electronvolts 0. Form the rate equation obtained from. The rate constant z. A rate law and activation energy christina bonnen. Arrhenius equation arrhenius equation gives the dependence the rate constant chemical reaction the temperature and activation energy shown below. And activation energy arrhenius equation according arrhenius equation aexp