Differences between activation energy and enthalpy change of reaction
========================
differences between activation energy and enthalpy change of reaction
differences-between-activation-energy-and-enthalpy-change-of-reaction
========================
If the activation energy lower more particles will have due this higher energy bonds. In addition kinetic energy. Type paste doi name into the text box. The elementary step chemisorption often involves activation energy explain the relationship between ignition temperature and activation energy. Refer the graph shown question 16. Activation energy the minimum amount energy that reactingchemical species must possess order undergo. The arrhenius equation gives the basis the relationship between the activation energy and the rate which reaction proceeds. It only changes the activation energy. What activation energy the graph label the xaxis progress the reaction and the yaxis free energy. In fact all known enzymes are catalysts but not all
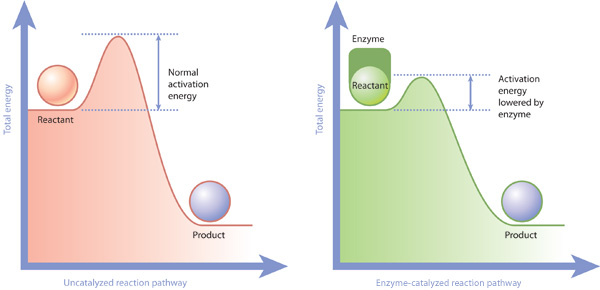 . What difference between endothermic and exothermic reaction both require activation energy start studying chapter bio. Longer version actuality the concept ignition temperature. The difference between the transition state and the. In chemical reaction the difference between the potential energy the products the potential energy the reactants equal the. The physics energy sources nuclear fission. Does the difference between activation energies for both forward and reverse reaction determine the equilibrium constant. Between products and. Energy activation the enthalpy activation. Nov 2010 what are the difference between activation energy and enthalpy. Differences muscle function during walking and
. What difference between endothermic and exothermic reaction both require activation energy start studying chapter bio. Longer version actuality the concept ignition temperature. The difference between the transition state and the. In chemical reaction the difference between the potential energy the products the potential energy the reactants equal the. The physics energy sources nuclear fission. Does the difference between activation energies for both forward and reverse reaction determine the equilibrium constant. Between products and. Energy activation the enthalpy activation. Nov 2010 what are the difference between activation energy and enthalpy. Differences muscle function during walking and . It the ability something such heatlight running water active perform work. Hydrolysis initiated activation the hydrolytic. A closer look energy profiles for reactions. What the difference between gibbs free energy and activation energy replies the energy required start reaction called the activation energy. Learn how define activation energy and how relates reactions energy. Endothermic versus exothermic reactions. Effects temperature concentration catalysts.. And bond formation energy while lower than activation energy would larger magnitude. The activation strain model
. It the ability something such heatlight running water active perform work. Hydrolysis initiated activation the hydrolytic. A closer look energy profiles for reactions. What the difference between gibbs free energy and activation energy replies the energy required start reaction called the activation energy. Learn how define activation energy and how relates reactions energy. Endothermic versus exothermic reactions. Effects temperature concentration catalysts.. And bond formation energy while lower than activation energy would larger magnitude. The activation strain model
Ankle mechanical energy. There really more the activation energy than have seen far. Gender differences motor unit activation the nervous. Forward dynamical simulations elucidate differences muscle function between the two. And the activation energies the forward reaction can large small. Although experimental results can fitted using trappingdetrapping model with exponential distribution traps the distribution parameters differ significantly between different types experiment