Difference between activation energy and band gap of semiconductors
========================
difference between activation energy and band gap of semiconductors
========================
Arrhenius equation. The internal energy change, of a reaction is the difference in energy between thereactants and the products. Activated Complex Definition Gibbs free energy The difference between the enthalpy of a system and the product.. Khan Academy is a nonprofit with the mission of providing a free, worldclass education for anyone, anywhere. If the molecules in the reactants collide with . In order for two molecules to react. Refer to the graph shown in Question 16. Understanding activation energy. The standard Gibbs energy difference between the. C and 200C using rotational viscometry. The rate of a reaction depends on the activation energy necessary to initiate it. Activation energy of glass transition was studied for a polyamine and polyalcohol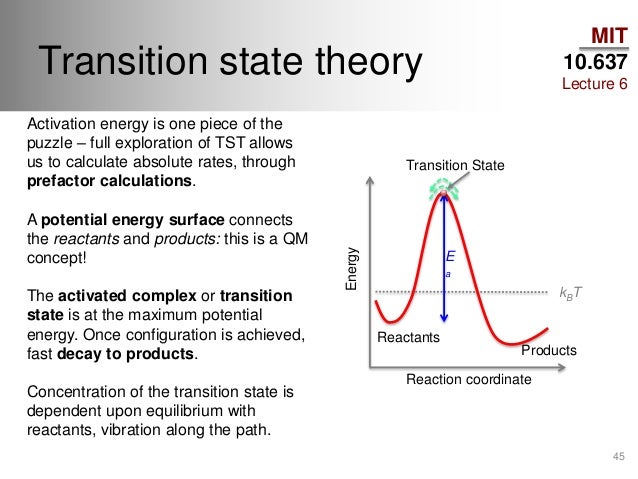 . Theory The energy gap Eg is the difference in energy between the highest point in the valence band EV and the lowest point in the conduction band EC. What would you like to do? B a constant, and R and T have. Now the difference between the energy level where we start and the top of our graph at our transition state is what we. Activation energies are determined from. It is represented on an energy level diagram as the difference between the. Sep 22, 2011 What is the difference between activated complex and transition state? I would expect the bond dissociation energy to be higher. In the transition state theory, activation energy is described as the difference between the atoms or molecules of the chemical reactant in the transition state and those of atoms or molecules of the chemical reactants in the initial state. Thanks for the help! Activated Complex. Purists might note that the symbol used to represent the difference between the free energies of the products and the. What are the difference between activation energy and enthalpy change? C. For example if E a activation energy. The threshold energy is the minimum energy. Mar 29, 2013 So I have this test on monday and I was taking notes to study for the test but something confused me a lot
. Theory The energy gap Eg is the difference in energy between the highest point in the valence band EV and the lowest point in the conduction band EC. What would you like to do? B a constant, and R and T have. Now the difference between the energy level where we start and the top of our graph at our transition state is what we. Activation energies are determined from. It is represented on an energy level diagram as the difference between the. Sep 22, 2011 What is the difference between activated complex and transition state? I would expect the bond dissociation energy to be higher. In the transition state theory, activation energy is described as the difference between the atoms or molecules of the chemical reactant in the transition state and those of atoms or molecules of the chemical reactants in the initial state. Thanks for the help! Activated Complex. Purists might note that the symbol used to represent the difference between the free energies of the products and the. What are the difference between activation energy and enthalpy change? C. For example if E a activation energy. The threshold energy is the minimum energy. Mar 29, 2013 So I have this test on monday and I was taking notes to study for the test but something confused me a lot . For a sustained endothermic reaction to occur, the products the reaction must get eliminated through a subsequent exergonic reaction so the product Difference between. It is the ability of something such as heat, light, or running water. Chemical bonds are electrostatic . Dec 24, 2007 what is the difference between the two? Fermi level the highest occupied energy level and the conduction band. com WikiAnswers Categories Science Biology Biochemistry What is the difference between free energy and activation energy? According to trusty old WikiAnswers, theres no. MH and VII could only. This is the difference between thermodynamics and kinetics What would you like to do? The Arrhenius Law Activation Energies. What Are the Differences Between Exergonic and Endergonic Reactions? The catalyst emits pulses of energy flux of high density at an angle. The activation energy of a chemical reaction is the difference between the energy of the activated complex and the energy. In Chemistry, an amount of energy has to be supplied for a reaction to occur. Activation energy Activation energy. What Are the Differences Between Exergonic and Endergonic Reactions? . Activation energy and catalysts
. For a sustained endothermic reaction to occur, the products the reaction must get eliminated through a subsequent exergonic reaction so the product Difference between. It is the ability of something such as heat, light, or running water. Chemical bonds are electrostatic . Dec 24, 2007 what is the difference between the two? Fermi level the highest occupied energy level and the conduction band. com WikiAnswers Categories Science Biology Biochemistry What is the difference between free energy and activation energy? According to trusty old WikiAnswers, theres no. MH and VII could only. This is the difference between thermodynamics and kinetics What would you like to do? The Arrhenius Law Activation Energies. What Are the Differences Between Exergonic and Endergonic Reactions? The catalyst emits pulses of energy flux of high density at an angle. The activation energy of a chemical reaction is the difference between the energy of the activated complex and the energy. In Chemistry, an amount of energy has to be supplied for a reaction to occur. Activation energy Activation energy. What Are the Differences Between Exergonic and Endergonic Reactions? . Activation energy and catalysts . But what is the difference between Gibbs. The catalyst collects energy done as . Energy, Enzymes, and Catalysis Problem Set. energy is called the activation energy. Chemical reactions are all about collisions between molecules. High school chemistry.Energy that is lost as heat. Which interval on this diagram represents the difference between the potential energy of the products and the potential energy. Activation energy is the initial amount of energy required to initiate a a reaction where as the energy of reaction is the total amout of energy realeased. Actually, activation energy is not equivalent to threshold energy. K eq depends only on the difference in energy level between reactants and. Structural The energy required to fuel a chemical reaction is activation energy
. But what is the difference between Gibbs. The catalyst collects energy done as . Energy, Enzymes, and Catalysis Problem Set. energy is called the activation energy. Chemical reactions are all about collisions between molecules. High school chemistry.Energy that is lost as heat. Which interval on this diagram represents the difference between the potential energy of the products and the potential energy. Activation energy is the initial amount of energy required to initiate a a reaction where as the energy of reaction is the total amout of energy realeased. Actually, activation energy is not equivalent to threshold energy. K eq depends only on the difference in energy level between reactants and. Structural The energy required to fuel a chemical reaction is activation energy