Cross Coupled

💣 👉🏻👉🏻👉🏻 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
www.seas.ucla.edu/brweb/papers/Journals/BR_Magzine1.pdf
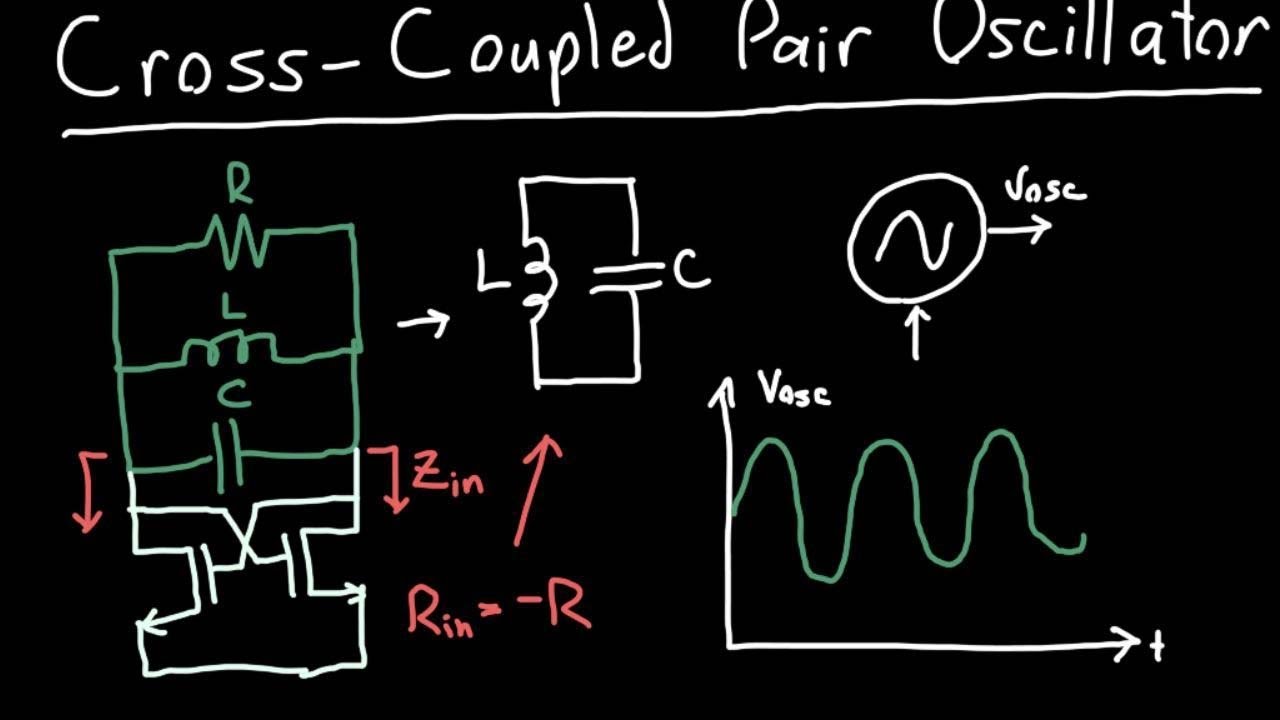
The cross-coupled pair (XCP) is such a topology: it has evolved for 95 years and adapted …
https://www.linguee.com/english-russian/translation/cross-coupled.html
Many translated example sentences containing "cross-coupled" – Russian-English dictionary and search engine …
https://en.m.wikipedia.org/wiki/Palladium-catalyzed_coupling_reactions
A cross-coupling reaction in organic chemistry is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bondin the product R-R'…
A cross-coupling reaction in organic chemistry is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations.
Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed cross coupling reactions.
https://m.youtube.com/watch?v=W8Wwv5_HrDs
Перевести · 08.03.2018 · https://www.patreon.com/edmundsjIf you want to see more of these …
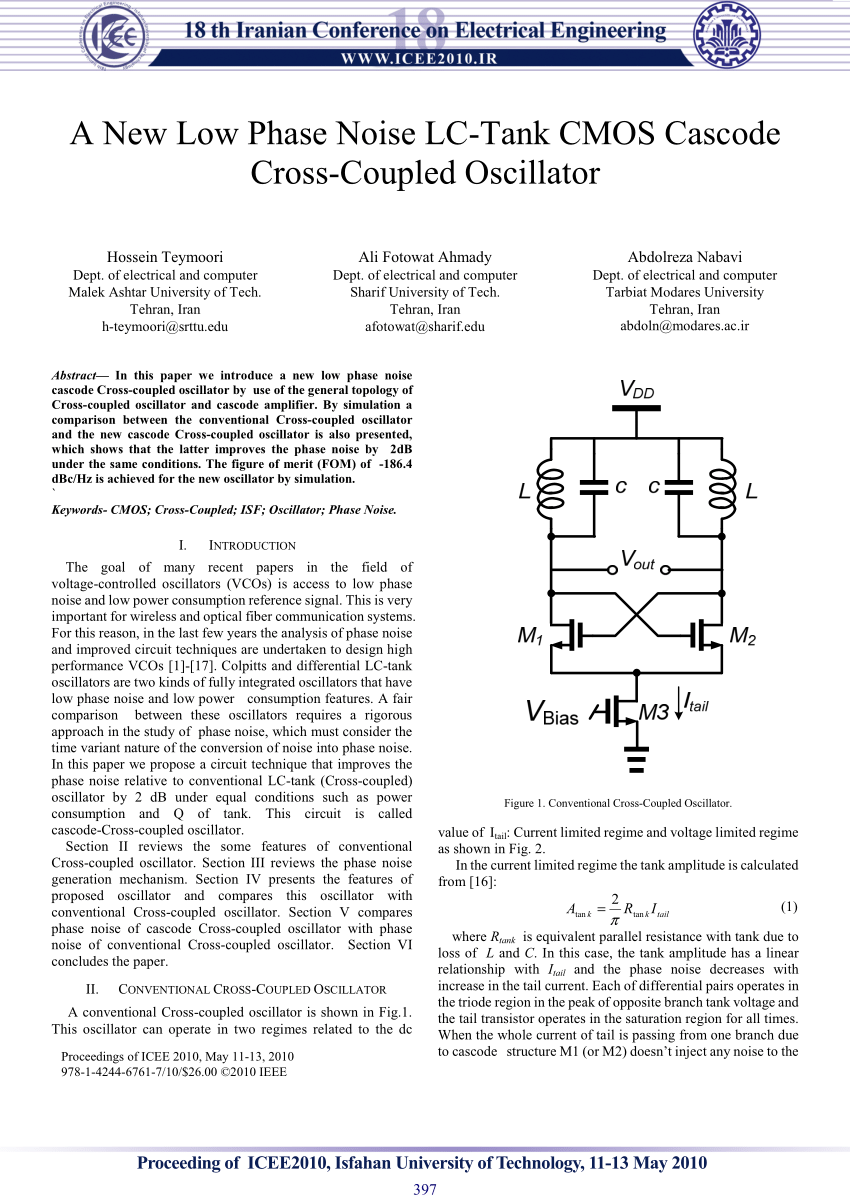
Cross coupled oscillator simulation in ADS + Phase noise analysis
Cross Coupled Pair Oscillator Part 2
Design Example: Nuhertz on Cross-Coupled Filters
YouTube › Organic Chemistry Explained!
Experimental Cross Coupled Shared Apex Loop Antenna (Part 3)
www.seas.ucla.edu/brweb/papers/Journals/BR_Magzine2.pdf
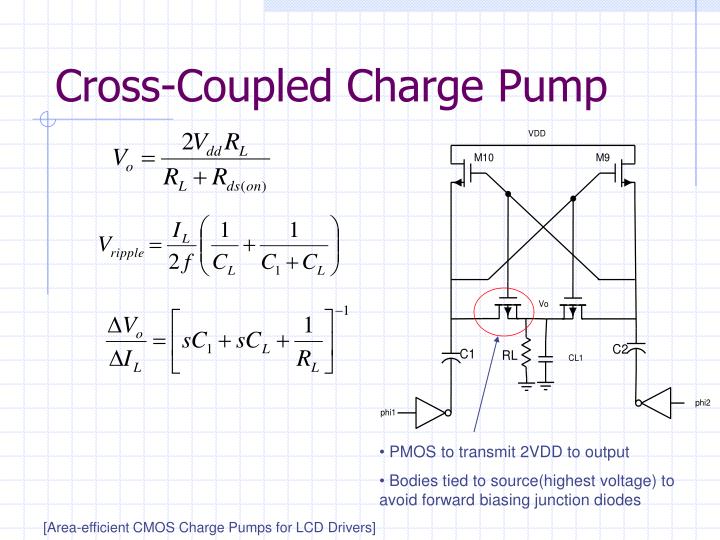
The Cross-Coupled Pair—Part II Digital Object Identifier 10.1109/MSSC.2014.2352532 Date of publication: 12 November 2014 Following a general overview of the cross-coupled …
https://www.quora.com/What-is-cross-coupling
Перевести · Cross coupling means an addition of extra capacitance by a net/signal(which toggles) passing close by to the signal desired. Cross …
The cross-coupled pair (XCP) is such a topology: it has evolved for 95 years and adapted itself to various device technologies, supply voltages, and operation speeds. In this and future columns, we analyze this circuit’s properties and study its applications in both analog and digital design.
www.seas.ucla.edu/brweb/papers/Journals/…
The feedback you provide will help us show you more relevant content in the future. Cross coupling means an addition of extra capacitance by a net/signal(which toggles) passing close by to the signal desired. Cross coupling caps add to degraded rise fall time, over/undershoots etc..
www.quora.com/What-is-cross-coupling
What is a cross coupled cavity filter?
What is a cross coupled cavity filter?
Welcome to CoupleFil Hompage For narrow single or multiple bandpass filters it is advantageous using cross-coupled filter designs. Since cross-couplings cause total reflexion for desired frequencies, a cross-coupled cavity filter needs fewer resonators than a simple-coupled one, what will result in a better through loss.
Which is the epitome of a cross coupled device?
Which is the epitome of a cross coupled device?
The epitome of a cross-coupled device is described like this. Arrange two transistors with bases returned to the positive supply, and collector load resistors arranged to drop half the supply voltage. These are two not unusual amplifiers.
www.quora.com/What-is-cross-coupling
https://m.youtube.com/watch?v=yS8qcKH-1aQ
Перевести · 08.03.2018 · https://www.patreon.com/edmundsjIf you want to see more of these …
https://m.youtube.com/watch?v=PlRtEWghX-0
Перевести · 08.03.2018 · https://www.patreon.com/edmundsjIf you want to see more of these …
Не удается получить доступ к вашему текущему расположению. Для получения лучших результатов предоставьте Bing доступ к данным о расположении или введите расположение.
Не удается получить доступ к расположению вашего устройства. Для получения лучших результатов введите расположение.
A cross-coupling reaction in organic chemistry is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'.[1][2][3] Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations.
The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequently, the second partner undergoes transmetallation with a source of R'−. The final step is reductive elimination of the two coupling fragments to regenerate the catalyst and give the organic product. Unsaturated organic groups couple more easily in part because they add readily. The intermediates are also less prone to beta-hydride elimination.[6]
Catalysts are often based on palladium, which is frequently selected due to high functional group tolerance. Organopalladium compounds are generally stable towards water and air. Palladium catalysts can be problematic for the pharmaceutical industry, which faces extensive regulation regarding heavy metals. Many pharmaceutical chemists attempt to use coupling reactions early in production to minimize metal traces in the product.[7] Heterogeneous catalysts based on Pd are also well developed.[8]
Copper-based catalysts are also common, especially for coupling involving heteroatom-C bonds.[9][10]
Iron-,[11] cobalt-,[12] and nickel-based[13] catalysts have been investigated.
The leaving group X in the organic partner is usually a halide, although triflate, tosylate and other pseudohalide have been used. Chloride is an ideal group due to the low cost of organochlorine compounds. Frequently, however, C–Cl bonds are too inert, and bromide or iodide leaving groups are required for acceptable rates. The main group metal in the organometallic partner usually is an electropositive element such as tin, zinc, silicon, or boron.
Many cross-couplings entail forming carbon–carbon bonds.
Cu-catalyzed version by Kochi, 1971
see Liebeskind–Srogl coupling, gives ketones
Many cross-couplings entail forming carbon–heteroatom bonds (heteroatom = S, N, O). A popular method is the Buchwald–Hartwig reaction:
N-C coupling,
second generation free amine
One method for palladium-catalyzed cross-coupling reactions of aryl halides with fluorinated arenes was reported by Keith Fagnou and co-workers. It is unusual in that it involves C–H functionalisation at an electron deficient arene.[17]
Cross-coupling reactions are important for the production of pharmaceuticals,[3] examples being montelukast, eletriptan, naproxen, varenicline, and resveratrol.[18] Some polymers and monomers are also prepared in this way.[6]
^ Organic Synthesis using Transition Metals Rod Bates ISBN 978-1-84127-107-1
^ New Trends in Cross-Coupling: Theory and Applications Thomas Colacot (Editor) 2014 ISBN 978-1-84973-896-5
^ a b King, A. O.; Yasuda, N. (2004). "Palladium-Catalyzed Cross-Coupling Reactions in the Synthesis of Pharmaceuticals". Organometallics in Process Chemistry. Topics in Organometallic Chemistry. 6. Heidelberg: Springer. pp. 205–245. doi:10.1007/b94551. ISBN 978-3-540-01603-8.
^ "The Nobel Prize in Chemistry 2010 - Richard F. Heck, Ei-ichi Negishi, Akira Suzuki". NobelPrize.org. 2010-10-06. Retrieved 2010-10-06. CS1 maint: discouraged parameter (link)
^ Johansson Seechurn, Carin C. C.; Kitching, Matthew O.; Colacot, Thomas J.; Snieckus, Victor (2012). "Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize". Angewandte Chemie International Edition. 51 (21): 5062–5085. doi:10.1002/anie.201107017. PMID 22573393. S2CID 20582425.
^ a b Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 1-891389-53-X
^ Thayer, Ann (2005-09-05). "Removing Impurities". Chemical & Engineering News. Retrieved 2015-12-11.
^ Yin, L.; Liebscher, J. (2007). "Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts". Chemical Reviews. 107 (1): 133–173. doi:10.1021/cr0505674. PMID 17212474. S2CID 36974481.
^ Corbet, Jean-Pierre; Mignani, Gérard (2006). "Selected Patented Cross-Coupling Reaction Technologies". Chemical Reviews. 106 (7): 2651–2710. doi:10.1021/cr0505268. PMID 16836296.
^ Evano, Gwilherm; Blanchard, Nicolas; Toumi, Mathieu (2008). "Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis". Chemical Reviews. 108 (8): 3054–3131. doi:10.1021/cr8002505. PMID 18698737.
^ Robin B. Bedford (2015). "How Low Does Iron Go? Chasing the Active Species in Fe-Catalyzed Cross-Coupling Reactions". Acc. Chem. Res. 48 (5): 1485–1493. doi:10.1021/acs.accounts.5b00042. PMID 25916260.
^ Cahiez, GéRard; Moyeux, Alban (2010). "Cobalt-Catalyzed Cross-Coupling Reactions". Chemical Reviews. 110 (3): 1435–1462. doi:10.1021/cr9000786. PMID 20148539.
^ Rosen, Brad M.; Quasdorf, Kyle W.; Wilson, Daniella A.; Zhang, Na; Resmerita, Ana-Maria; Garg, Neil K.; Percec, Virgil (2011). "Nickel-Catalyzed Cross-Couplings Involving Carbon−Oxygen Bonds". Chemical Reviews. 111 (3): 1346–1416. doi:10.1021/cr100259t. PMC 3055945. PMID 21133429.
^ Murahashi, Shunichi; Yamamura, Masaaki; Yanagisawa, Kenichi; Mita, Nobuaki; Kondo, Kaoru (1979). "Stereoselective synthesis of alkenes and alkenyl sulfides from alkenyl halides using palladium and ruthenium catalysts". The Journal of Organic Chemistry. 44 (14): 2408–2417. doi:10.1021/jo01328a016. ISSN 0022-3263.
^ Jennifer X. Qiao; Patrick Y.S. Lam (2011). "Recent Advances in Chan–Lam Coupling Reaction: Copper-Promoted C–Heteroatom Bond Cross-Coupling Reactions with Boronic Acids and Derivatives". In Dennis G. Hall (ed.). Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. Wiley-VCH. pp. 315–361. doi:10.1002/9783527639328.ch6. ISBN 9783527639328.
^ Ruiz-Castillo, P.; Buchwald, S. L. (2016). "Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions". Chemical Reviews. 116 (19): 12564–12649. doi:10.1021/acs.chemrev.6b00512. PMC 5070552. PMID 27689804.
^ M. Lafrance; C. N. Rowley; T. K. Woo; K. Fagnou (2006). "Catalytic Intermolecular Direct Arylation of Perfluorobenzenes". J. Am. Chem. Soc. 128 (27): 8754–8756. CiteSeerX 10.1.1.631.607. doi:10.1021/ja062509l. PMID 16819868.
^ Cornils, Boy; Börner, Armin; Franke, Robert; Zhang, Baoxin; Wiebus, Ernst; Schmid, Klaus (2017). "Hydroformylation". Applied Homogeneous Catalysis with Organometallic Compounds. pp. 23–90. doi:10.1002/9783527651733.ch2. ISBN 9783527328970.
Content is available under CC BY-SA 3.0 unless otherwise noted.
Bbw Celebrity
Couple Up
Milfs Sex New
Big Milf Hd
Celebrity Mother
The Cross-Coupled Pair—Part I A
cross-coupled - Russian translation – Linguee
Cross-coupling reaction - Wikipedia
The Cross-Coupled Pair—Part II
What is cross-coupling? - Quora
Cross Coupled