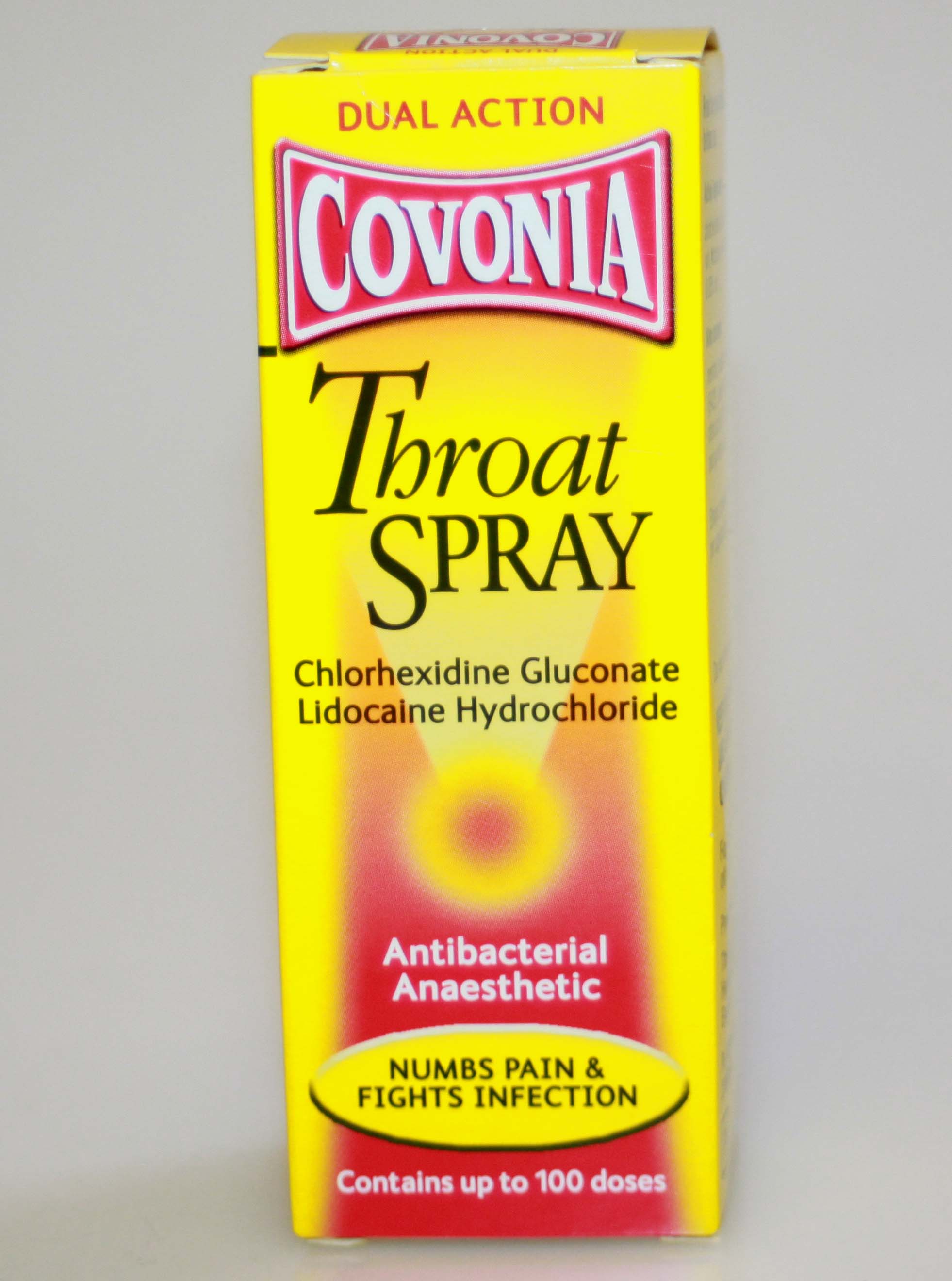
Covonia Throat Spray

🛑 👉🏻👉🏻👉🏻 INFORMATION AVAILABLE CLICK HERE👈🏻👈🏻👈🏻
We earn a commission for products purchased through some links in this article.
Available over the counter from pharmacies, we explain everything you need to know about this sore throat treatment
Medically reviewed by Dr Roger Henderson and words by Helen Marshall, BPharm, MRPharmS
Covonia throat spray used for relieving the pain of sore throats or minor throat infections. This medicine is suitable for adults and children aged 12 years and over.
Here's everything you need to know about covonia throat spray, including how it works, who shouldn't use it and the potential side effects:
Covonia throat spray contains two active ingredients, lidocaine and chlorhexidine.
Lidocaine is a type of medicine called a local anaesthetic. It numbs pain in the area where you apply it by temporarily blocking the pathway of pain signals along nerves.
Advertisement - Continue Reading Below
Chlorhexidine is an antiseptic and disinfectant agent that kills various bacteria, viruses and fungi that can cause throat infections. It helps kill the germs that may be associated with causing your sore throat.
Certain people shouldn't use Covonia throat spray. They include:
Meanwhile, who should see a doctor before using Covonia throat spray?
You should see a doctor if your sore throat is very severe or has lasted longer than two days, if you are also feeling sick or vomiting, or if you have a fever (high temperature) or headache with your sore throat.
The following people should also see a doctor before using Covonia throat spray:
Robitussin mucus cough and congestion relief
Adults and children aged 12 years and over should use three to five sprays for each dose. This can be repeated 6 to 10 times a day as needed. If you still have a sore throat after two days you should see your doctor. The spray should not be used continuously on a regular basis.
To use the spray, raise the spray nozzle from vertical to horizontal position. Hold your head upright and open your mouth. Point the nozzle at the back of your throat and press the spray button three to five times.
⚠️ Do not exceed the recommended dose.
After each dose turn the spray nozzle back down to close the spray. If the spray doesn't seem to work the nozzle may have become blocked. You should remove the spray nozzle from the pump and clean it in a cup of hot water for a few minutes. Allow it to dry before fixing it back onto the pump.
Do not use it unless you have checked with your doctor first.
Although lidocaine and chlorhexidine have been in widespread use for many years without apparently causing harm, their safety during pregnancy and breastfeeding has not been specifically studied so you should get advice from your doctor before using Covonia throat spray.
Medicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that may rarely be associated with Covonia throat spray. Just because a side effect is stated here doesn't mean that all people using Covonia throat spray will experience that or any side effect.
Read the leaflet that comes with the medicine or talk to your pharmacist if you want any more information about the possible side effects of Covonia throat spray. If you think you have experienced a side effect, did you know you can report this using the yellow card website?
It's unlikely that Covonia throat spray will affect any other medicines when it's used for short periods of time. However, if you're already taking any medicines, including those bought without a prescription and herbal medicines, then make sure you tell your pharmacist before you start using this medicine, so they can check that the combination is safe.
It's fine to use Covonia throat spray alongside painkillers such as paracetamol or ibuprofen.
Benylin chesty coughs original facts
Dr Roger Henderson Dr Roger Henderson is a Senior GP, national medical columnist and UK medical director for LIVA Healthcare He appears regularly on television and radio and has written multiple books.
This content is created and maintained by a third party, and imported onto this page to help users provide their email addresses. You may be able to find more information about this and similar content at piano.io
Advertisement - Continue Reading Below
What is Sudafed decongestant tablets and liquid?
Sudafed blocked nose spray (xylometazoline)
©2021 Hearst UK is the trading name of the National Magazine Company Ltd, 30 Panton Street, Leicester Square, London, SW1Y 4AJ. Registered in England. All Rights Reserved.
About Netdoctor
Disclaimer
Terms & Conditions
Privacy Notice
Cookies Policy
Contact
Complaints
Sitemap
Advertising
Cookies Choices
Hearst and third parties use cookies and similar technologies (“Cookies”) on this site. Some Cookies are necessary to make this site and our content available to you. Other Cookies analyse and measure audience and traffic. Cookies are also used by us and third parties such as advertisers, ad-tech providers and others (“Vendors”) to develop and serve ads more relevant to your interests based on your consent or our legitimate interests. For a list of Vendors that can set Cookies on your device or browser when you interact with this site and the purposes for which Cookies are set by Vendors and us, click Learn More below. From time to time we may add or remove Vendors and/or Cookies. You can adjust your preferences including your right to object where legitimate interest is used, or withdraw your consent to certain Cookies at any time. We process personal data obtained through the use of Cookies (such as a cookie identifier and/or IP address) for the purposes described in the Privacy Notice and Cookies Policy published on the site. To consent to the use of Cookies and proceed to the site, click Accept below.
Actively scan device characteristics for identification. Store and/or access information on a device. Select basic ads. Create a personalised ads profile. Select personalised ads. Create a personalised content profile. Select personalised content. Measure ad performance. Measure content performance. Apply market research to generate audience insights. Develop and improve products.
List of IAB Vendors
Covonia Sore Throat 0.2/0.05%w/v Oromucosal Spray Lemon Flavour
Active ingredient
chlorhexidine gluconate
lidocaine hydrochloride
Last updated on emc: 13 Jul 2021
View changes
Print
This information is intended for use by health professionals
Covonia Sore Throat 0.2/0.05%w/v Oromucosal Spray Lemon Flavour
2. Qualitative and quantitative composition
Lidocaine hydrochloride monohydrate 0.05% w/v
For the full list of excipients, see section 6.1.
Covonia Sore Throat Spray is indicated for the symptomatic relief of painful, irritated sore throats.
It is a sugar free preparation and can be used by diabetics.
Additional therapy is required in the event of bacterial infection accompanied by fever.
4.2 Posology and method of administration
The dose is 3 to 5 sprays (0.3 - 0.5 ml). This can be repeated 6 to 10 times per day.
Known hypersensitivity to the product or any of its components, especially in those with a history of possible chlorhexidine-related allergic reactions (see sections 4.4 and 4.8).
4.4 Special warnings and precautions for use
Treatment with Covonia Sore Throat Spray should be limited to the relief of existing pain and irritation when strictly necessary. It is not intended for prolonged use, either continuously or repeatedly.
Ingredients with specified warnings
This medicine contains 100mg propylene glycol per 5 sprays (0.5ml).
This medicine contains 120mg of alcohol (ethanol) per 5 sprays (0.5ml). The amount in 5 sprays of this medicine is equivalent to less than 3ml of beer or 2ml of wine. The small amount of alcohol in this medicine will not have any noticeable effects.
Covonia Sore Throat Spray contains chlorhexidine. Chlorhexidine is known to induce hypersensitivity, including generalised allergic reactions and anaphylactic shock. The prevalence of chlorhexidine hypersensitivity is not known, but available literature suggests this is likely to be rare. Covonia Sore Throat Spray should not be administered to anyone with a potential history of allergic reaction to chlorhexidine-containing compound (see sections 4.3 and 4.8).
Do not use and consult your doctor if you have difficulty in swallowing. Do not use without consulting your doctor if sore throat is severe or has lasted for more than 2 days or is accompanied by high fever, headache, nausea or vomiting.
4.5 Interaction with other medicinal products and other forms of interaction
Chlorhexidine is not known to interact with other drugs.
Whilst a number of interactions are theoretically possible with lidocaine these drug interactions are not likely to be clinically relevant to the use of Covonia Sore Throat Spray which is administered topically. Concomitant therapy with drugs that reduce hepatic blood flow (e.g. propranolol, cimetidine) may reduce the clearance of lidocaine. Long term administration with drugs that induce drug-metabolising microsomal enzymes (e.g. phenytoin, barbiturates) may increase dosage requirements of lidocaine. The cardiac depressant effects of lidocaine are additive with those of beta blockers and other anti-arrhythmics (e.g. mexiletine). Lidocaine is a weak inhibitor of pseudocholinesterase and may prolong the action of suxamethonium. Hypokalaemia produced by acetazolamide, loop diuretics and thiazides may antagonise the effect of lidocaine.
4.6 Fertility, pregnancy and lactation
There is inadequate evidence of the safety of lidocaine and chlorhexidine in human pregnancy but they have been in wide use for many years without apparent ill consequence. Covonia Sore Throat Spray should only be used in pregnancy and breast feeding under the direction of a physician.
Lidocaine is excreted in breast milk but in such small quantities that there is generally no risk of the infant being affected at therapeutic dose levels.
4.7 Effects on ability to drive and use machines
Chlorhexidine and lidocaine are usually well tolerated and no unwanted effects have been reported for the product during local short term use.
In extremely rare cases local anaesthetic preparations have been associated with allergic reactions. Hypersensitivity reactions to lidocaine hydrochloride following local injection have presented as localised oedema with slight difficulty in breathing or as a generalised rash.
Chlorhexidine may sometimes produce discoloration of the teeth and tongue. This is not permanent and disappears after treatment when chlorhexidine is discontinued. Occasionally, a dental scale and polish may be necessary to remove the stain completely. Skin sensitivity to chlorhexidine has occasionally been reported, and severe hypersensitivity reactions, including anaphylactic shock, have been reported on rare occasions following the topical use of chlorhexidine. Chlorhexidine may cause transient taste disturbances, a burning sensation of the tongue, and occasional parotid gland swelling.
Frequency not known: Allergic skin reactions such as dermatitis, pruritus, erythema, eczema, rash, urticaria, skin irritation, and blisters.
Frequency not known: Hypersensitivity including anaphylactic shock (see sections 4.3 and 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme: www.mhra.gov.uk/yellowcard or search MHRA yellow Card in the Google Play or Apple App Store.
The use of oral topical anaesthetic agents may interfere with swallowing and thus enhance the danger of aspiration of food in the respiratory tract. To this end, overdosage with Covonia Sore Throat Spray (use of 10 ml or more) could produce a slight risk of inducing too great a local anaesthetic effect in the glottis region and consequent reduction in control of the swallowing reflex.
Excessively high blood concentrations of lidocaine may produce CNS and/or cardiovascular effects. Early CNS effects may consist of nervousness, dizziness, tinnitus, nystagmus, restlessness, excitation, paraesthesia, blurred vision, nausea, vomiting, and tremors which may progress to medullary depression and tonic and clonic convulsions. Cardiovascular reactions are depressant and may be characterised by hypotension, myocardial depression, bradycardia and possibly cardiac arrest.
Although the bioavailability of lidocaine is low it may be sufficient to result in significant toxicity when swallowed. CNS toxicity, seizures and death have been reported following the ingestion of topical preparations. However, in the case of Covonia Sore Throat Spray more than one litre would have to be swallowed to be equivalent to the ingestion of sufficient lidocaine (0.5 g or more) to cause significant toxicity.
Systemic toxicity from chlorhexidine is rare. The main consequence of ingestion is mucosal irritation.
Treatment of lidocaine overdosage consists of ensuring adequate ventilation and arresting convulsions. Ventilation should be maintained with oxygen by assisted or controlled respiration as required. Convulsions may be treated with thiopentone, diazepam or succinylcholine. As succinylcholine will arrest respiration it should only be used if the clinician has the ability to perform endotracheal intubation and to manage a totally paralysed patient. If ventricular fibrillation or cardiac arrest occurs, effective cardiovascular resuscitation must be instituted. Adrenaline in repeated doses and sodium bicarbonate should be given as rapidly as possible.
Lidocaine is a local anaesthetic of the amide type. Like other local anaesthetics, lidocaine impairs the generation and conduction of the nerve impulses by slowing depolarisation. This results from blocking of the large transient increase in permeability of the cell membrane to sodium ions that follows initial depolarisation of the membrane. Lidocaine also reduces the permeability of the resting axon to potassium and to sodium ions.
Chlorhexidine is a bisbiguanide antiseptic and disinfectant which is bactericidal or bacteriostatic against a wide range of Gram-positive and Gram-negative bacteria. It is more effective against Gram-positive than Gram-negative bacteria and some species of Pseudomonas and Proteus have low susceptibility. It is relatively ineffective against mycobacteria. It inhibits some viruses and is active against some fungi.
Covonia Sore Throat Spray is applied topically for local action in the throat. On swallowing the spray solution or saliva, small amounts may reach the digestive system and some may be absorbed from the oral and pharyngeal mucosa.
Lidocaine is readily absorbed from oral mucous membranes, the gastrointestinal tract and through damaged skin. Absorption through intact skin is poor. Presystemic metabolism is extensive and bioavailability is only about 35% after oral administration. Following absorption, lidocaine is rapidly distributed to all body tissues. It crosses the placenta and blood-brain barrier. Approximately 65% is bound to plasma protein. It has a plasma half-life of 1.6 hours. Lidocaine is largely metabolised in the liver. Any alteration in liver function or hepatic blood flow can have a significant effect on its pharmacokinetics and dosage requirements. Metabolism in the liver is rapid and approximately 90% of a given dose is dealkylated to form monoethylglycinexylidide (MEGX) and glycinexylidide (GX); both of which may contribute to its therapeutic and toxic effects. Further metabolism occurs and metabolites are excreted in the urine with less than 10% of unchanged lidocaine.
Chlorhexidine is poorly absorbed from mucous membranes, intact skin and the gastrointestinal tract. It is only minimally metabolised by the liver and is excreted through bile. Urinary excretion is very low.
There are no further relevant data.
Use within 6 months of first opening
6.4 Special precautions for storage
6.5 Nature and contents of container
30ml: Brown, type III glass (E.P.) bottle with a polypropylene/polyethylene metering pump and nozzle.
6.6 Special precautions for disposal and other handling
8. Marketing authorisation number(s)
9. Date of first authorisation/renewal of the authorisation
Last updated on emc: 13 Jul 2021
View changes
Print
Company contact details
Thornton & Ross Ltd
Linthwaite, Huddersfield, West Yorks, HD7 5QH
To bookmark a medicine you must sign up and log in.
To email a medicine you must sign up and log in.
To view the changes to a medicine you must sign up and log in.
To find similar products you must sign up and log in.
By clicking “Accept All Cookies”, you agree to the storing of cookies on your device to enhance site navigation, analyse site usage, and assist in our marketing efforts. Cookie Policy
Outdoor Erotic Pictures
Hd Video Nipples Sucking
Silk Stockings Film
Tan Solo Tu
Kiss Mature Torrent
Covonia Throat Spray - Summary of Product Characteristics ...
Covonia | Sore Throat Oromucosal Spray | Sugar Free
Covonia Sore Throat 0.2/0.05%w/v Oromucosal Spray Lemon ...
Covonia Throat Spray - Product - tabletwise.net
Review of Covonia Dual Action Throat Spray - trusted reviewer
Amazon.co.uk: covonia throat spray
Covonia Throat Spray – 30ml - Medicine Marketplace
Buy Covonia Throat Spray | Chemist Direct
Covonia Throat Spray


























.jpg%3fv%3d2b83ee9b%26amp%3bmode%3dv)