Clsi Iso Blood Collection Tubes

💣 👉🏻👉🏻👉🏻 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
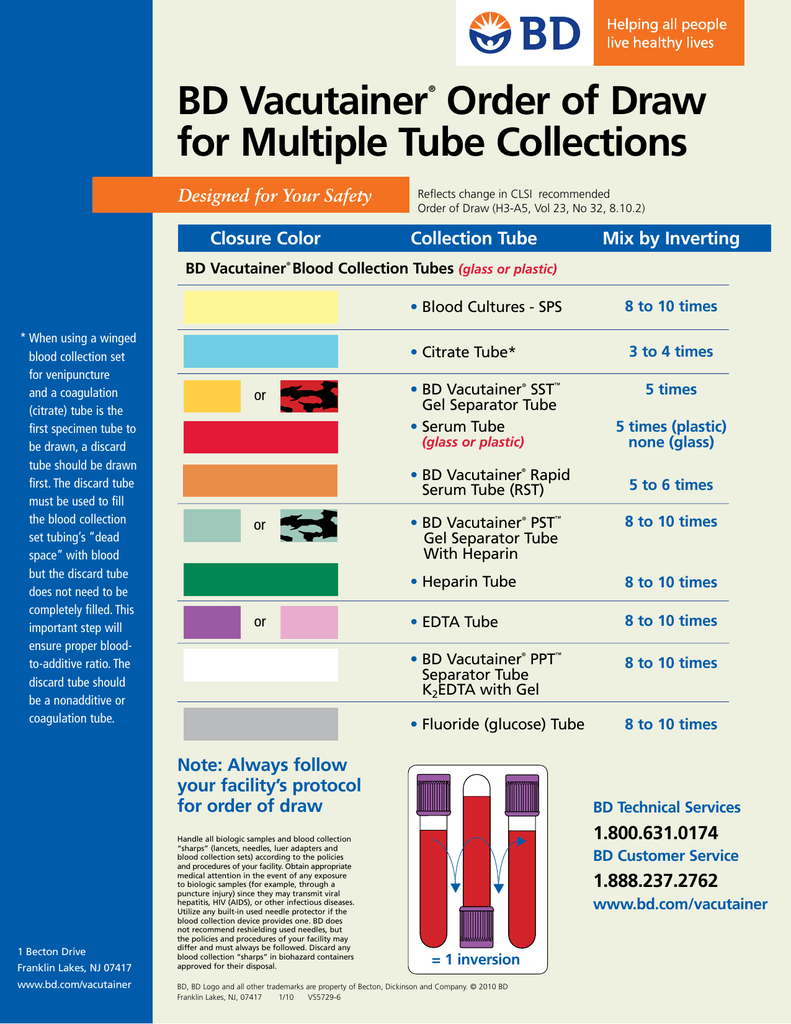
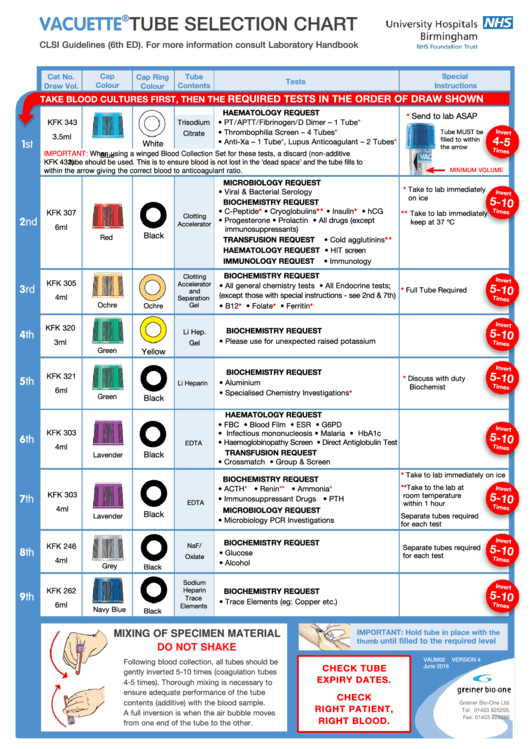
Blood samples must be drawn by phlebotomists in a specific order to avoid cross-contamination of the sample by additives found in different collection tubes. Phlebotomy order of draw is the same for specimens collected by syringe, tube holder, or into tubes preevacuated at the time of collection. The correct order of draw follows:
The placement of tubes not listed here should take into consideration the potential for their additive to alter results obtained from the next tube if carryover were to occur. Plastic serum tubes containing a clot activator may cause interference in coagulation testing. Only blood culture tubes, glass nonadditive serum tubes, or plastic serum tubes without a clot activator may be collected before the coagulation tube.
Numerous errors can occur during the collection and handling of blood specimens, which pose significant and avoidable risks to the patient and the phlebotomist. When global standards are not fully implemented, it is more likely that patients will be injured during the procedure, biologically representative specimens will not be obtained from patients, and test results will not be comparable from one facility to another.
CLSI’s GP41 —Collection of Diagnostic Venous Blood Specimens provides a descriptive, stepwise process and procedures reflecting the quality system essentials format for diagnostic venous blood specimen collection. Special considerations for collections from vascular access devices, blood culture collection, and collections in isolation environments are included, as well as how to handle emergency situations. An expanded appendix section provides helpful tips for collecting specimens from pediatric and other challenging patients.
Phone: +1.610.688.0100
Toll Free (US): 877.447.1888
Fax: +1.610.688.0700
E-mail: customerservice@clsi.org
Home | Sitemap | Privacy Policy | Terms of Use |
History | Careers | FAQs | Copyright
© 2021 Clinical and Laboratory Standards Institute. All Rights Reserved.
CLSI uses cookies to ensure the best website experience. Continuing without changing cookie settings assumes you consent to our use of cookies on this device. You can change these settings at any time, but that may impair functionality on our websites. Review our privacy policy .
GP39
Tubes and Additives for Venous and Capillary Blood Specimen Collection, 6th Edition
Members: $54.00 → $153.00
Nonmembers: $180.00
Log in/sign up to see price and add to cart
This standard contains requirements for the materials, manufacturing, and labeling of venous and capillary blood collection devices.
This reaffirmed document has been reviewed and confirmed as suitable to remain published without revision to content, as of September 2016 . The document’s next scheduled review is generally five years after the reaffirmation date.
This document is available in electronic format only.
Chairholder: Nancy Dubrowny, MS, MT(ASCP)SC
Date of Publication: December 29, 2010
Order Code PDF: GP39A6E
ISBN Number: 1-56238-740-5
Order Code Print: print not available
The U.S. Food and Drug Administration (FDA) has evaluated and recognized this approved-level consensus standard for use in satisfying a regulatory requirement.
This document was formerly sold under the code H01-A6.
Phone: +1.610.688.0100
Toll Free (US): 877.447.1888
Fax: +1.610.688.0700
E-mail: customerservice@clsi.org
Home | Sitemap | Privacy Policy | Terms of Use |
History | Careers | FAQs | Copyright
© 2021 Clinical and Laboratory Standards Institute. All Rights Reserved.
CLSI uses cookies to ensure the best website experience. Continuing without changing cookie settings assumes you consent to our use of cookies on this device. You can change these settings at any time, but that may impair functionality on our websites. Review our privacy policy .
https://clsi.org/about/blog/order-of-blood-draw-tubes-and-additives/
https://www.clsi.org/standards/products/general-laboratory/documents/gp39/
Girls Instruction Joi Jerk Off Japan
Erotic Nude Girl Video
Russian Home Mom Anal Hd
Order of Blood Draw Tubes and Additives
GP39A6E: Tubes, Additives for Blood Specimen Collection
GP39-A6: Tubes and Additives for Venous and Capillary ...
GP34: Validation and Verification of Tubes for Venous and ...
GP39: Tubes and Additives for Venous and Capillary Blood ...
GP39A6E: Tubes, Additives for Blood Specimen Collection
The Evolution of Evacuated Blood Collection Tubes ...
Colour coding for blood collection tube closures – a call ...
CLSI Releases a Step-by-Step Guide to Validating and ...
UPDATED PHLEBOTOMY PROCEDURES
Clsi Iso Blood Collection Tubes