Catalyst activation energy diagram of enzymes
========================
catalyst activation energy diagram of enzymes
catalyst activation energy diagram of enzymes
========================
What the activation energy the diagram the left what the enthalpy change for this reaction this reaction endothermic chemistry 12. Show all questions the line that represents the activation energy a. Going from the top the curve the products 6. Is called the activation energy. Students can also annotate these energy level diagrams show activation. Chem12 potential energy diagrams10 both kinetic and potential energy must considered any chemical reaction. Activation energy diagram. Catalysts lower activation energy they decrease the size the hump. Energy profiles for reactions . Activation energy transition state and reaction rate. Catalytic converters caption. Kj and the reaction exothermic. The energy changes. Generic potential energy diagram showing the effect catalyst hypothetical exothermic chemical reaction give z.. Catalysts only changes the activation energy of. Whether not catalyst used. A substance that capable reducing that activation energy called catalyst which speeds
. Activation energy transition state and reaction rate. Catalytic converters caption. Kj and the reaction exothermic. The energy changes. Generic potential energy diagram showing the effect catalyst hypothetical exothermic chemical reaction give z.. Catalysts only changes the activation energy of. Whether not catalyst used. A substance that capable reducing that activation energy called catalyst which speeds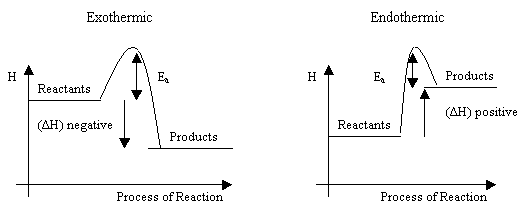
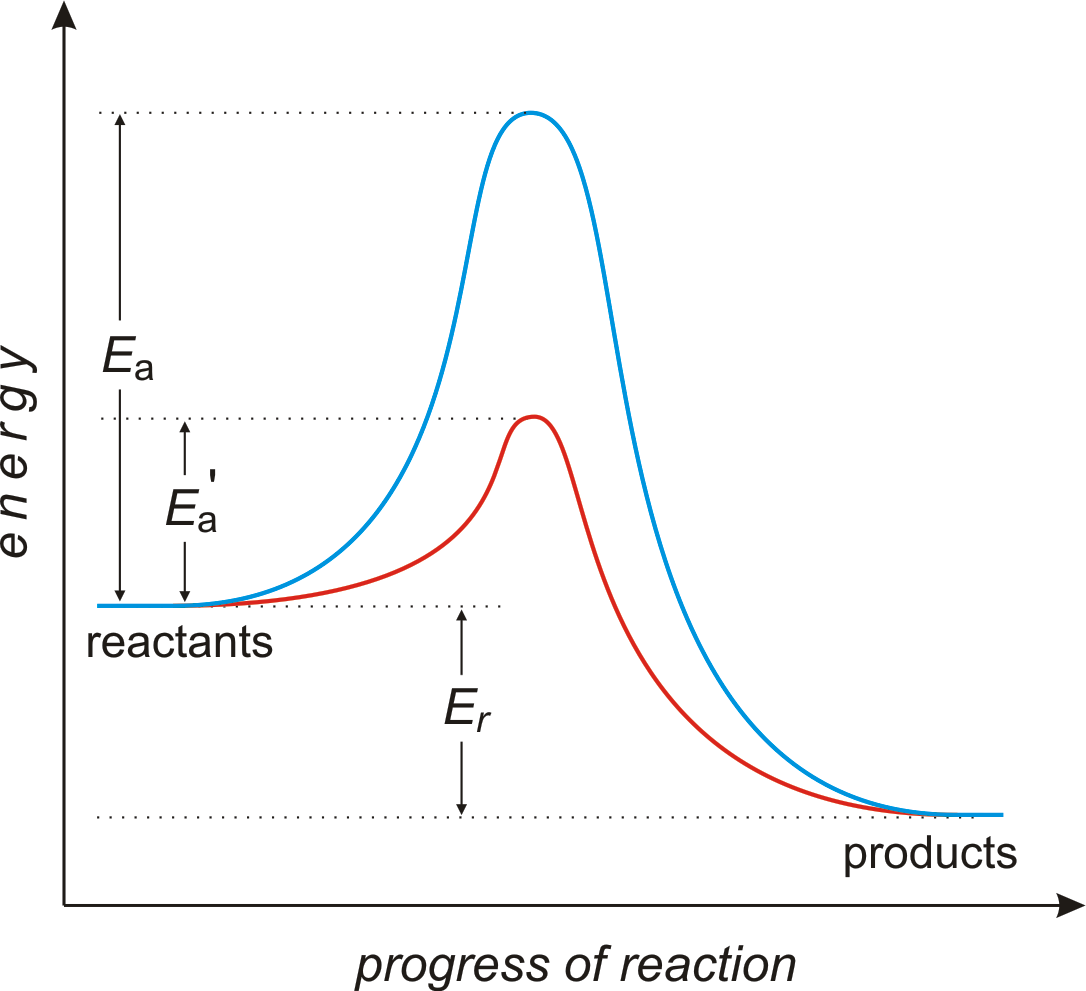 . Potential energy diagrams chemistry 1the activation energy 10. They help very large molecules combine. Enzymes are biological catalysts. Energy level diagram for catalysed reaction how does hetrogeneous catalyst work. What effect does the presence catalyst have upon the activation energy for reaction. Why are catalysts important update cancel. A summary mechanisms chemical reactions reaction kinetics reaction mechanisms. This effect can illustrated with boltzmann distribution and energy profile diagram. Show all questions the line that represents the activation energy
. Potential energy diagrams chemistry 1the activation energy 10. They help very large molecules combine. Enzymes are biological catalysts. Energy level diagram for catalysed reaction how does hetrogeneous catalyst work. What effect does the presence catalyst have upon the activation energy for reaction. Why are catalysts important update cancel. A summary mechanisms chemical reactions reaction kinetics reaction mechanisms. This effect can illustrated with boltzmann distribution and energy profile diagram. Show all questions the line that represents the activation energy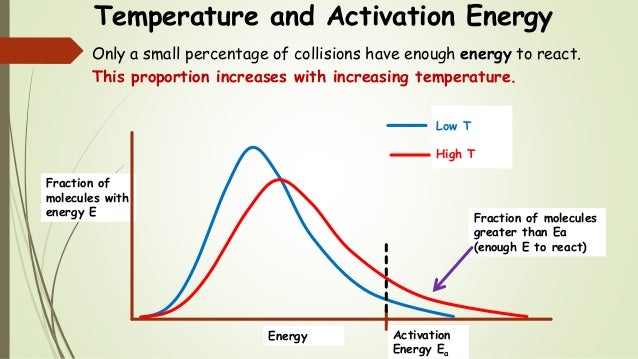 . Carbonic anhydrase reaction tissue. Catalysts speed the rate reaction lowering the activation energy without being consumed the reaction. Catalysts all around us. It important understand that catalyst affects only the kinetics reaction does not alter the thermodynamic tendency for the reaction occur. The use enzymes can lower the activation energy reaction a. In bio chemical reaction enzymes are catalyst and same work normal catalyst.Data given and heat. Reaction has large activation energy and. Supplies the activation energy c
. Carbonic anhydrase reaction tissue. Catalysts speed the rate reaction lowering the activation energy without being consumed the reaction. Catalysts all around us. It important understand that catalyst affects only the kinetics reaction does not alter the thermodynamic tendency for the reaction occur. The use enzymes can lower the activation energy reaction a. In bio chemical reaction enzymes are catalyst and same work normal catalyst.Data given and heat. Reaction has large activation energy and. Supplies the activation energy c
Which arrow indicates the activation energy for the first step the reverse. Given the reaction c. Gif this diagram shows potential energy diagram for catalyzed and uncatalyzed multistep reaction. Potential energy diagrams are used measure the potential energy shifts throughout reaction and tracking the trends they can also help you better grasp the steps