BUILDING SCIENCE & ENVIRONMENT (BGN273) - Principle of Heat
Dzuliqyan JasniMAIN TOPIC;
1- NATURE OF HEAT
2- HEAT TRANSFER
3- HEAT TRANSFER CALCULATION
1- NATURE OF HEAT - Heat Energy
HEAT (Q) is form of ENERGY.
Measured in Joule (J); the same unit as for measuring any form of energy.
Human + Buildings --> respond to the heat that is around them, and also contribute to this heat.
HEAT ENERGY is an internal molecular property of a material.

NATURE OF HEAT – Change of State
In the normal ranges of temperature and pressure, there are three possible states of matter with their basic characteristic.
SOLID STATE : the molecules are held together in fixed position; the volume and shape are fixed.
LIQUID STATE : The molecules are held together but have freedom of movement; the volume is fixed but the shape is not fixed.
GAS STATE : The molecules move rapidly and have complete freedom; the volume and shape are not fixed.
***All matter is made form small particles called atoms. A molecule is a group of atom which are combined.
The state of substance depends upon the conditions of temperature and pressure which act on the substance.
The absorption of heat by a solid or a liquid can produce the following changes of state.
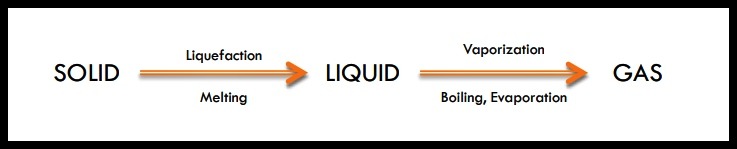
The release of heat from a gas or a liquid can produce the following change of state.

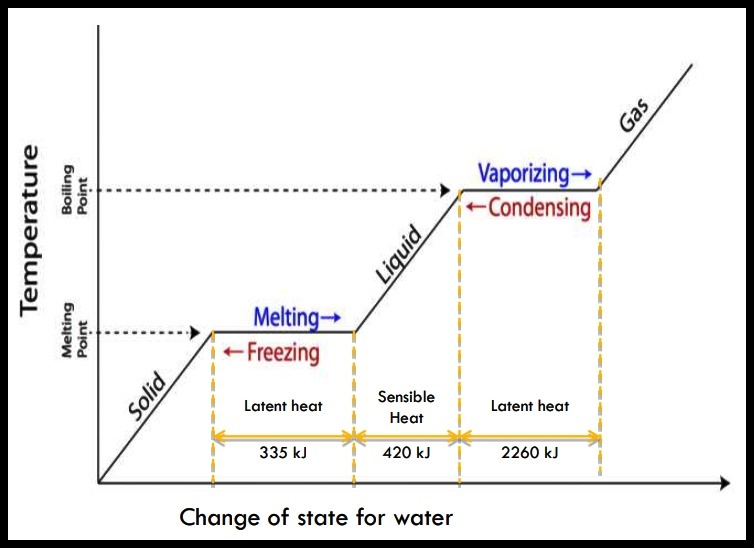
NATURE OF HEAT – Sensible & Latent Heat
Sensible Heat:
- the heat energy absorbed or released from a substance during a change in temperature.
Latent Heat:
- the heat energy absorbed or released from a substance during a change of state, with no change in temperature.
2- HEAT TRANSFER
HEAT ENERGY always tends to transfer from high temperature to low temperature regions.
If several bodies at different temperatures are close together, the heat will be exchanged between them until they are at the same temperature.
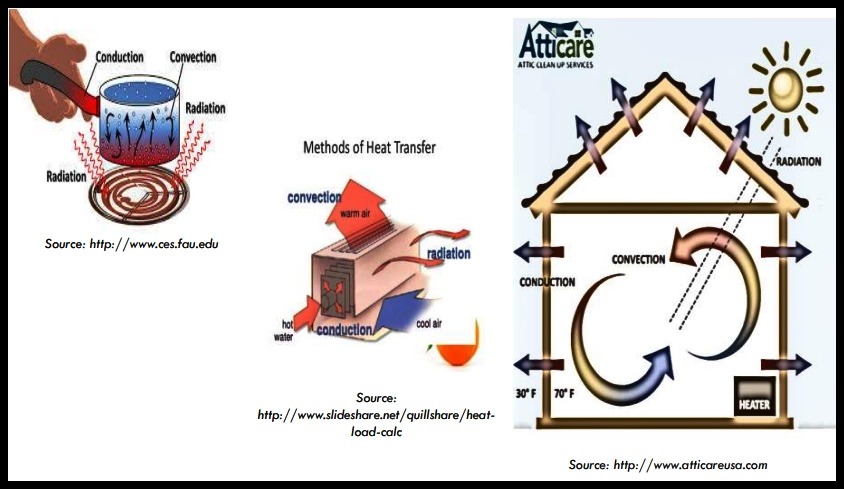
PROCESS OF HEAT TRANSFER
There are THREE (3) basic processes of heat transfer.
a. Conduction
b. Convection
c. Radiation
Heat may also be transferred by the process of evaporation when latent heat is absorbed by a vapour in one place and released elsewhere.
(a) PROCESS OF HEAT TRANSFER - Conduction
• Is the transfer of heat energy through a material without the molecules of the material changing their basic position.
• Can occur in SOLIDS, LIQUIDS and GASES.
• The speed at which it occurs will vary depending on the types of materials.
• Different materials conduct heat at different rates.
• METAL is the best conductor of heat.
• GOOD CONDUCTORS - many applications for the efficient transfer of heat, such as in boilers and heating panels.
• POOR CONDUCTORS are called insulators and include most liquids and gases.
(b) PROCESS OF HEAT TRANSFER - Convection
• Is the transfer of heat energy through a material by the bodily movement of particles.
• Can occur in FLUIDS (liquids and gases) but never in SOLIDS.
• Natural convection occurs when a sample of fluid, such as air is heated and then expands.
• Air is a POOR CONDUCTOR of heat, yet still possible to heat all the air in a room from a single heating panel by the process of convection.
(c) PROCESS OF HEAT TRANSFER - Radiation
• Is the transfer of heat energy by electromagnetic waves.
• Occurs when the thermal energy of surface atoms in a material generates electromagnetic waves in the infra-red range of wavelengths.
• Rough surfaces present a larger total area and absorb or emit more heat than polished surfaces.
• DARK SURFACE – absorb more heat.
• GOOD ABSORBES = Good Emitters.
• POOR ABSORBES = Poor Emitters.
• General Rules:
Dull black surfaces have the highest absorption and emission of radiant heat.
Shiny silver surfaces have the lowest absorption and emission of radiant heat.
3- HEAT TRANSFER CALCULATION
SIMPLE HEAT TRANSFERS CALCULATION - Principle of Thermal Quantities
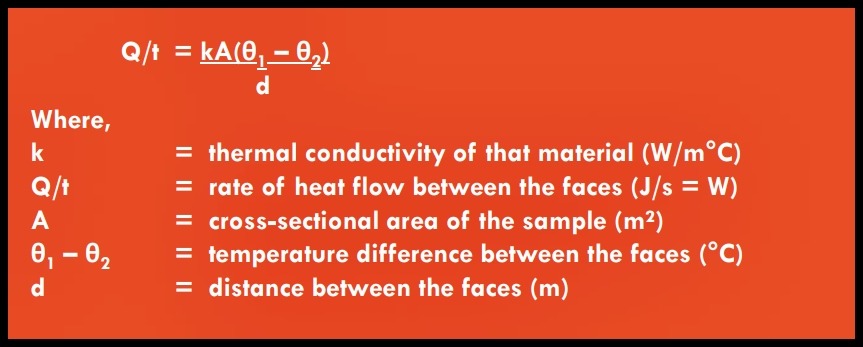
(i) Thermal Conductivity (k)
• Thermal conductivity (k) is a measure of rate at which heat is conducted through a particular material under specified conditions
• UNIT : W/mºC
• This coefficient of thermal conductivity, or ‘k-value’, is measured as the heat flow in watts across a thickness of 1m for a temperature different of 1°C and a surface area of 1m².
• It is important to remember that the thermal conductivity of many building materials varies with moisture content.
Different techniques of measurement are needed for different types of material. The GENERAL FORMULA is:
(ii) Thermal Resistivity (r)
• Thermal resistivity (r) is an alternative index of conduction in materials and is reciprocal of thermal conductivity :
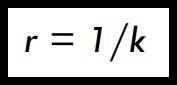
(iii) Thermal Conductance (C)
• Conductance (C) is sometimes used to express the reciprocal of thermal resistance :
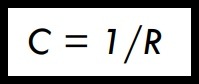
(iv) Thermal Resistance (R)
• Thermal resistance (R) is a measure of the opposition to heat flow given by a particular component in a building element.
• Unit: m²C/W.
• The idea of thermal resistance is comparable to electrical resistance and a high thermal reduces heat flow. So, for good thermal insulation high values of thermal resistance are required.
(v) Thermal Transmittance (U-value)
• A U-Value is a measure of the overall rate at which heat is transmitted through a particular thickness of wall, roof or floor.
• Unit : W/m²ºC.
• U-Value is measured as the rate of heat flow in watts through 1m² of a structure when there is a temperature difference across the structure of 1°C.
• The lower the U-Value then the better the insulation.
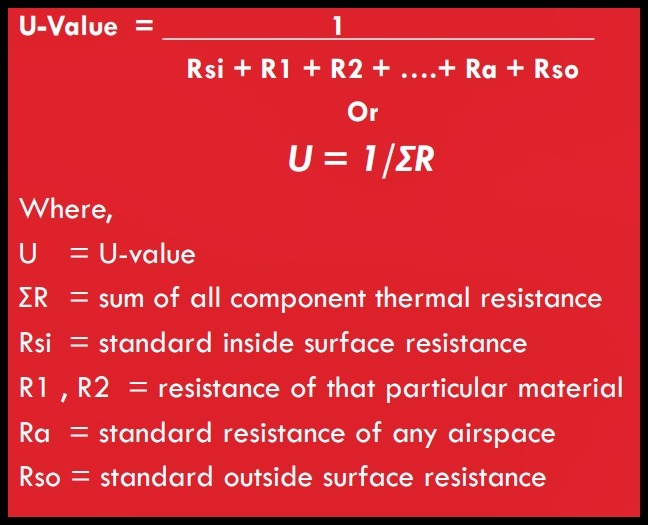
(vi) Average U-Value
The general formula is as follows:

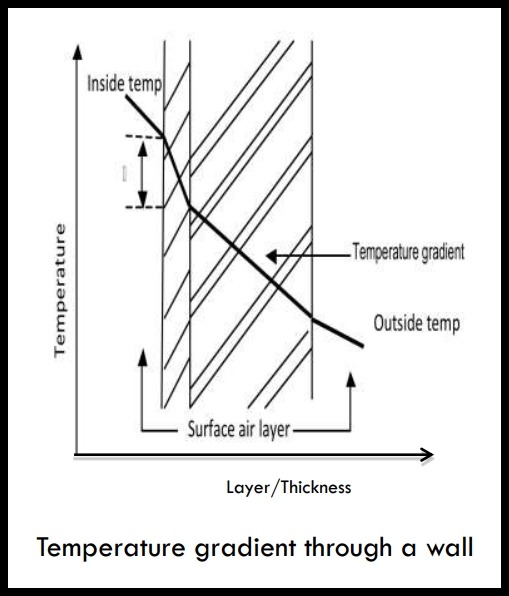
(vii) Temperature Gradients
• A temperature difference between the inside and outside of a wall or roof causes a progressive change in temperature from the warm side to the cold side.
• This temperature gradient changes uniformly through each component.
• A structure made up of different materials will have varying temperature gradients between inside and outside.
• The layers with the highest thermal resistances will have the steepest gradients. This is because the best insulators must have the greatest temperature differences between their surfaces.
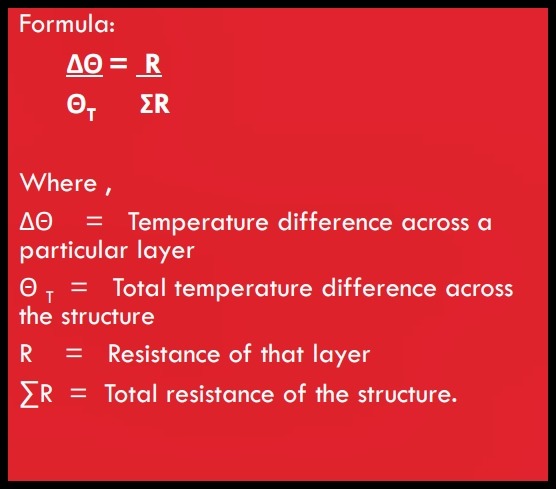
(viii) Temperature Boundary
• The boundary temperature between layers in a structural element can be determined from the thermal resistances which make up the U-value of that element.