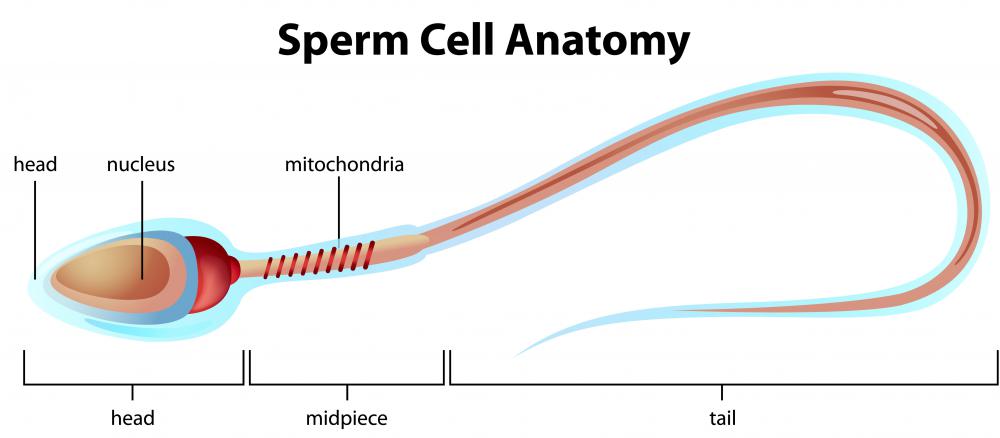
Artificial Sperm

🔞 ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
Artificial Sperm
We reserve the right to remove any content at any time from this Community, including without limitation if it violates the Community Standards . We ask that you report content that you in good faith believe violates the above rules by clicking the Flag link next to the offending comment or by filling out this form . New comments are only accepted for 3 days from the date of publication.
Oldest
Oldest Most Liked Most Replies Most Active Editor's Pick
Subscribe
Why Subscribe?
Subscription Bundles
Digital Subscriptions FAQs
Gift Subscriptions
Home Delivery
ePaper
eBooks
Crosswords
Newspaper Archive
Email Alerts & Newsletters
Article Archive
Executive Jobs
Page Sales
Photo Sales
About Us
Advertise
Contact Us
The Irish Times Trust CLG
Careers
Our Partners
Rewarding Times
MyHome.ie
Irish Racing
Top 1000
MyAntiques.ie
The Gloss
Irish Times Training
Terms & Conditions
Privacy Policy
Cookie Information
Cookie Settings
Community Standards
Copyright
FAQs
In 2016, a 24-year-old woman gave birth to her first child in the Portland hospital in London. Rashid was a perfect boy, weighing in at 3.2kg. His mother, Moaza Al Matrooshi , was captivated, describing him as a “miracle”, a declaration not unusual among besotted first-time parents.
But she had more reason than most to make it.
Her son began as a sliver of tissue taken from an ovary removed from her body when she was nine years old. As Al Matrooshi went on to have life-saving chemotherapy to treat an inherited blood disorder, her ovarian tissue was mixed with cryoprotective agents and reduced a temperature of minus 196C, before being stored under liquid nitrogen. Back in 2001, there can have been little certainty that any of this would one day result in a baby, but it was her mother who insisted doctors try.
Her mother’s determination paid off. Fourteen years is a long time in the science of baby making.
In 2015, now in her early 20s and already beginning to enter menopause, Al Matrooshi travelled to Denmark to have the frozen ovarian tissue transplanted back into her body, and attached to her remaining left ovary.
Three months later, she began getting her period again. And a year later, Rashid was born.
Cryopreservation of ovarian tissue – or simply, ovary freezing – is one of a range of pioneering treatments that is giving new hope to women, and even prepubescent girls, who are worried about preserving their fertility. Unlike egg freezing, it has the potential to restore the ovarian endocrine function, and reset the entire female reproductive cycle, reversing menopause in women such as Al Matrooshi.
It is still early days, cautions Prof Mary Wingfield , the clinical director of Merrion Fertility Clinic and a consultant at the National Maternity Hospital in Dublin. “It’s still regarded that, for women with cancer, egg freezing is the most tried and tested method,” she says, and it is also the best chance for women who are about to undergo chemotherapy of later having a baby. As yet, ovarian freezing is not available in Ireland , but worldwide it has already resulted in about 100 births.
This is not the only recent innovation in the area of assisted reproduction. Some – such as the expansion in our understanding of the role played by sperm, and the ways in which is can be damaged by environment, lifestyle and ageing – are so fundamental it seems astonishing that science is only getting around to them now.
Others, such as the use of artificial intelligence in fertility labs to assess an embryo’s likelihood of becoming a baby, could prove truly revolutionary. And some – such as efforts to grow artificial sperm and artificial eggs – are probably best regarded as moonshots.
We are at “a hugely exciting moment” in the treatment of fertility, says Sheena Lewis , emeritus professor at Queen’s University Belfast and chair of the British Andrology Society .
FOR PEOPLE STRUGGLING to have a child of their own, it must seem that the pace of innovation in scientific advances in assisted reproduction has been punishingly slow. “There has been a kind of stagnation in developments. Most of the major developments in fertility treatment happened more than 10 years ago,” says Dr Bart Kuczera , a fertility consultant and gynaecologist from Beacon Care Fertility.
Patient safety has improved considerably over the last decade, which is no small achievement, he says, but there have been few big, headline breakthroughs.
“We’ve been at this for 42 years and we’ve fallen way behind,” says Lewis, pointing to reports from the European Society for Human Reproduction and Embryology’s 2019 annual meeting in Vienna earlier this summer, which suggested that although in-vitro fertilisation (IVF) use in Europe has risen, its success rate – and that of intracytoplasmic sperm injection (ICSI) – has plateaued.
And though the so-called take-home baby rate for couples going through IVF has improved since its early days, it remains stubbornly below 30 per cent. Most experts regard even this oft-cited figure as an almost uselessly crude statistic.
“IVF and assisted reproduction is full of figures, and they can be very misleading,” says Wingfield. The under 30 per cent refers “to what people call the live birth rate, or the ‘take home baby’ rate after one treatment. On average, with one treatment, 28 per cent of people will be successful. But if they’re willing to do a few treatments, that goes up to 70 to 80 per cent. In women under 35, they have a 50 per cent chance of having a baby with one treatment in our clinic. But if she’s 42, that’s down to 5 per cent.”
The low success rates in women over 35 comes against a backdrop of couples delaying parenthood for a variety of individual and societal reasons, and a troubling, and so far unexplained, global rise in infertility rates among men. What’s happening to sperm will become a major area of research over the next decade.
“There has been a 50 per cent decline in male sperm count since the 1970s. And we aren’t fully able to discern why. It’s probably related to industrialisation, western lifestyles, endocrine disrupters, rising obesity. All of this is feeding into an increased need for assisted reproductive technologies. Coupled with that, the definite trend for couples to commence conception and start families later in life is having knock-on effects,” says consultant urologist and andrologist Ivor Cullen .
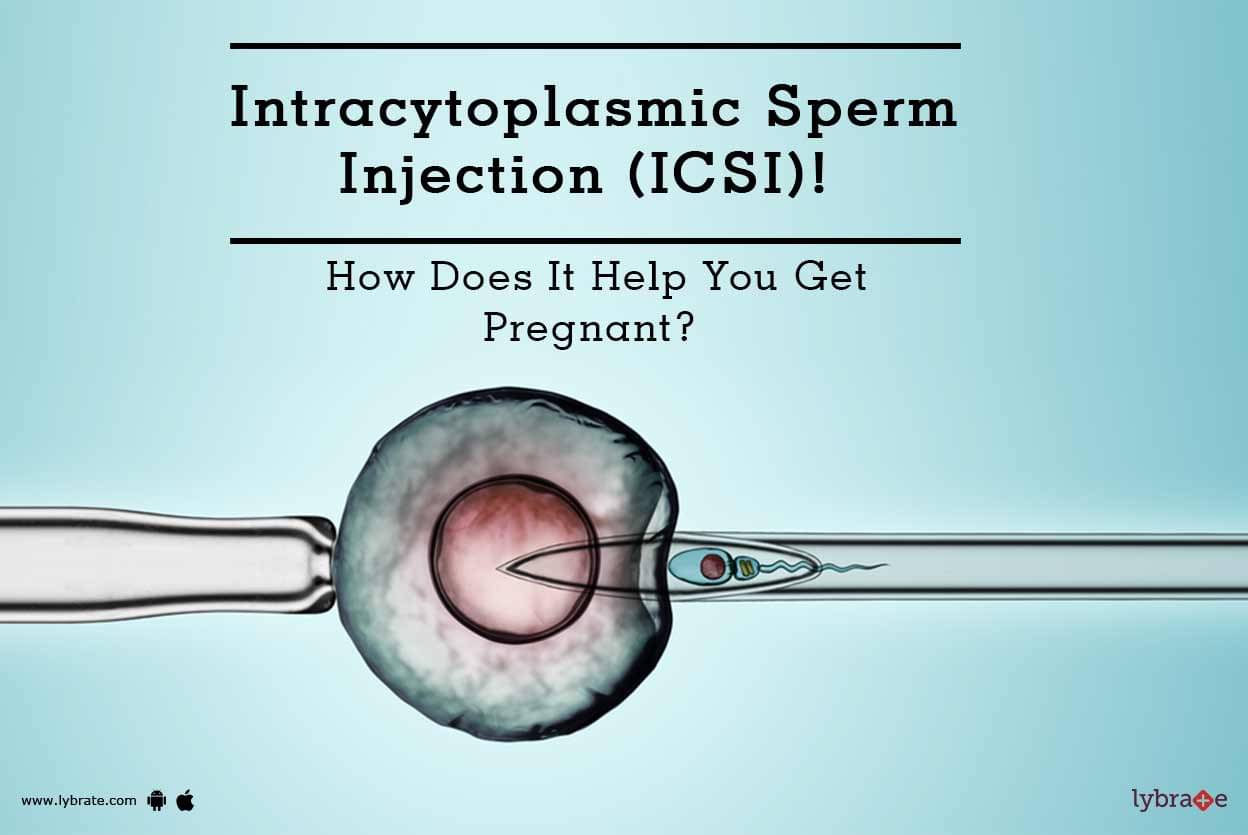
In the face of all this, science hasn’t moved nearly fast enough. The last truly revolutionary advance in assisted reproduction came as the result of a clumsy accident in a lab in Belgium in 1992. Doctors who were trying to inject a sperm into the space between an egg’s outer and inner membranes inadvertently plunged it straight into the egg instead. Previously, scientists had injected entire sea urchin sperm into sea urchin eggs, and nothing much had happened. It was expected that nothing much would happen here either.
But something did happen. The egg began to divide. It changed into an embryo, and then a foetus, and then a baby. And eventually that technique was used to create hundreds of thousands more babies.
ICSI, as it became known, offers any man who produces sperm, even if their sperm count is really low, a chance at fatherhood. And it is so effective that it is now used three times as often as IVF – despite little evidence for its use in couples where male-factor infertility is not the issue.
In fact, ICSI has done such a good job that researchers seem to have stopped looking for the reasons behind male-factor infertility altogether. ICSI “slowed us down in many ways from looking at male fertility. We didn’t ask questions like, why are sperm counts dropping? It is easy to say it’s diet and lifestyle, it’s high-fructose corn syrup, it’s pollution. But this will be a huge area for study and for research. Sperm are far more complex than we used to think,” says Dr John Kennedy , medical director at the Sims Fertility Clinic .
The first report in 1987 of a global decline in sperm counts of about 50 per cent in 50 years were so shocking that researchers immediately rushed to debunk it, says Lewis. “But then a couple of years ago, another paper was published that confirmed all the data.”
Research published in 2017 in the journal Human Reproduction Update revealed that sperm counts in men from America, Europe, Australia and New Zealand fell by more than 50 per cent in less than 40 years, in concentration and total count. But “it doesn’t mean male fertility has dropped by 50 per cent”, she adds. The real worry is not falling numbers “because most men produce a reasonable number of sperm anyway”. It is declining quality. “What we seem to be doing is creating a lifestyle and an environment which is really hazardous for sperm quality.”
Meanwhile, Lewis points out, the bulk of the research, messaging and the treatment of fertility over the last four decades – since the first IVF baby, Louise Brown , was born in 1978 – has been targeted at women. “You can’t really blame obstetricians, but the whole of the fertility market and treatment has been based on obstetrics and gynaecology. These doctors are all focused on women; that’s how they’ve been trained. Historically, we’ve thought the only thing that could be wrong with men was impotence.” If they weren’t impotent, the assumption went, they had to be fertile.
When men were tested – and it often didn’t happen until women had already been put through months of invasive treatment – they took semen analysis tests, which look at the number of sperm in an ejaculate; what they look like, also known as morphology; and how quickly they swim, or their motility.
Now, thanks to work done by Lewis and her colleagues at Queens, and other experts internationally, we know that these tests may have been missing out on the most important measure of all: the quality of DNA in that sperm. We now understand, says Lewis, “the only thing that matters once the sperm enters the egg is the DNA”.
Lewis is at the forefront of research into sperm DNA fragmentation, a term used to denote abnormal genetic material within the sperm, which has been linked to infertility and miscarriage. She developed a now widely-used test, the SpermComet test, which can measure DNA fragmentation. If sperm DNA is badly damaged, simple lifestyle changes – stopping smoking, not taking drugs, losing weight – can reverse it in as quickly as three months.
Meanwhile, other research has shown a link between advanced paternal age and a range of psychiatric, social and educational problems in their offspring.
All in all, it is clear that men have a biological clock – and that clock is ticking.
The next stage in fertility treatment should involve educating men on things such as sperm DNA. It will also involve making urologists and andrologists more integral to the fertility process, predicts Lewis.
There have been other advances in our understanding of potentially reversible causes of male infertility, says Cullen. Improvements in medications and supplements that benefit in sperm production; relatively simple surgical procedures, such as microsurgically treating varicoceles (essentially, varicose veins in the testicles, that are recognised to be a cause of compromised sperm production and decreased sperm quality); and more complex ones, such as microsurgical testicular-sperm extraction, or MicroTese, are all becoming integral to managing male-factor infertility.
MicroTese, which is used to treat infertility caused by non-obstructive azoospermia – essentially, a condition in which no live sperm are found in a semen sample – was first performed by Ivor Cullen in Ireland in 2016, and since then, he says, “it has been performed approximately 40 times, resulting in five live births and one ongoing pregnancy”.
It has, he says, changed the landscape, offering hope to couples where previously it was not possible to have a child with the father’s DNA.
But all of these treatments can only do so much to counteract the social and biological factors impacting on fertility. Over the next decade, both men and women need to be educated on the need to have their children earlier, ideally in their early 30s. “It’s not fair and reasonable to tell somebody who doesn’t want to have children that getting pregnant now is your best shot to have children, but sometimes that is the best advice. I wish we had a greater awareness about fertility. I wish guys were getting their semen analysis checked, and girls were getting their egg reserves checked,” says Kennedy.
Apps and home-testing kits that offer women a way of tracking their fertile cycles can be useful, but sometimes they just add more stress to an already stressful experience. “I think it’s good for people to know if they’re ovulating, but I’m not really in favour of everyone using apps,” says Wingfield.
“There have been studies showing they’re not really that accurate. If a woman has a regular cycle and it’s fairly regular – within a week of a 28-day cycle – she’s almost certainly ovulating. People can get too caught up in apps and ovulation kits.”
Several of the experts who spoke to The Irish Times were sceptical, too, about multinationals offering egg freezing to young women as a way to preserve their fertility.
“They are trying to buy the time during their most fertile period from younger women,” says Kuczera. “Yes, it gives you a chance in the future, when maybe you can’t afford it now; yes, it can protract your natural fertility beyond what you might otherwise expect.” But, he says, “How do you look at that, only that the company doesn’t want you to be pregnant?”
Kennedy says that, despite its “huge potential”, it is unlikely that egg freezing will live up to the hype generated by the breathless news reports of the past few years about companies such as Facebook and Google offering it to employees. And “the fundamental reason it’s not going to live up is that it’s not typically a 28-year-old woman – who’s going to have the best chance of success – who freezes her eggs. Instead, it’s a 38-year-old woman who hasn’t yet decided what she wants to do with regard to parenthood. The results are going to be much worse.”
Apart from the biological considerations, “do we really want to be having our children at 40 and 50, so that we’re 70 and 80 by the time they’ve left home?” asks Wingfield.
“There are a whole lot of societal issues that I don’t think people are thinking through.”
MANY OF THE MOST PROMISING developments in fertility treatment the next few years may not be headline-grabbing, but they have the potential to quietly revolutionise assisted reproduction – and present us with new social, moral and ethical dilemmas.
Artificial intelligence (AI) is already being used in fertility labs to help doctors decide which embryos to implant. “We are getting better and faster at testing embryos and minding embryos,” says Kennedy.
“The one thing we’ve been less good at is saying which embryo is going to give you a baby and which isn’t. It was a bit of a lucky dip.”
But all that is changing. In April this year, a study was published in NPJ Digital Medicine, in which scientists at Cornell University described training a Google deep-learning algorithm to identify 12,000 IVF embryos from photos as either good, fair, or poor. The system, nicknamed Stork, predicted what a team of human embryologists would say about the embryos with 95.7 per cent accuracy.
The most consistent volunteer embryologist in the study matched Stork’s results only 70 per cent of the time; the least, 25 per cent. In other words, “it’s kicking the embryologists’ asses. It is looking at things that we weren’t even looking at. It is looking at millions of data points, and it doesn’t have bad days or good days. That’s something that’s going to be huge in the future,” says Kennedy.
The Sims Fertility Clinic has its own deep-learning model, called IVY, using time-lapse videos to assess embryos in its clinic. “Whilst it’s not making decisions as to which embryos we use, we’re going to be part of a large multicentre trial to determine its validity in a prospective fashion,” Kennedy says.
Over the next decade, thanks to AI and advances in gene editing, embryo screening for genetic defects is likely to become entirely non-invasive, predicts Kuczera.
“The future brings the beauty and the beast. The beauty is that we can exclude embryos that are not viable. The technology is already available to substitute genes that cause a variety of conditions. But the beast is that people might use it, and overuse it, for other kinds of improvements. We already have the problem of sports doping in society. You can imagine what could be done with gene editing. You could produce a so-called superhero,” he says.
“You know the movie Gattaca? That’s where we’re headed,” says Kennedy. “It won’t just be about editing out genes for cystic fibrosis. We’ll get to a place where we will have the ability to create designer human beings – cutting out male pattern baldness, heart disease and so on.”
These advances pose “huge ethical dilemmas and moral dilemmas” that our legislative framework is nowhere near ready to tackle, Kennedy believes. “We’re busy talking about donor anonymity, when that conversation is over already. Everybody who is the product of donor sperm or a donor egg is going to find out.”
DNA analysis sites such as 23andme mean “the notion that will you be able to donate anonymously is nonsense. If you donate sperm or eggs, the bottom line is that you could get a knock on the door in 20 years’ time.”
The wider availability of fertility treatment services, combined with changing social attitudes, has already opened up the possibility of all kinds of new family formations that might have been unimaginable when Louise Brown was a baby: same-sex parents; transgender parents; surrogate parents; significantly older parents; families created with the help of donor sperm and eggs. And over the next 40 years, further leaps in science and technology are likely to offer unparalleled promise – and new, unimagined ethical dilemmas.
One of the first hints of what these dilemmas might looked like emerged from China in 2016. Researchers there reported that they had successfully created baby mice using artificial sperm. The scientists at the Chinese Academy of Sciences Institute of Zoology used embryonic stem cells – which are capable of becoming any type of tissue – to create immature sperm cells called spermatids. These were injected into mouse eggs, which fertilised and developed into healthy pups.
This procedure is a long way off being tested on human stem cells. But when that day comes, could it mean that “the end of men” – a phrase coined by Hanna Rosin to describe the end of the patriarchy – becomes a biological possibility?
“We’re miles and miles away from using it in people,” says Cullen. “But if we have the ability to turn stem cells into sperm cells, yes, it would revolutionise the landscape. For same-sex couples, you could potentially have two females successfully fertilising an egg without any male involved.”
And that’s not the only recent leap that could revolutionise how babies are made in the 21st century. In 2018, a group of researchers in Japan managed to turn human blood into stem cells, which they developed into immature human eggs.
The technology is in its infancy, but it has already raised the spectre of babies being created from the cells of, for example, other children, or even deceased people. “A woman might want to have George Clooney’s baby,” Ronald Green , a professor of religion and bioethicist at Dartmouth University told NPR radio in the US earlier this year. “And his hairdresser could start selling his hair follicles online. So we suddenly could see many, many progeny of George Clooney without his consent.”
Leaving aside the sceptre of a rogue hairdresser breeding an army of mini George Clooneys , it is certainly true that some of these advances will bring with them new, and so far unimagined, moral and ethical dilemmas. “Ireland will have to get its act together and move with the times,” says Cullen.
In the meantime, almost all of the fertility experts interviewed by The Irish Times offered the same response when they were asked what was the one thing they wished people understood about their fertility: that, for both men and women, it has a best-before date.
The bottom line, says Kuczera, is that “biology hasn’t changed for millennia, whereas lifespan and social evolution has, stretching childhood right out. If you ask women who are 35 to 40 when youth ends, they will say it ends in the late 30s. But unfortunately our biology doesn’t agree.” And, for now, there’s no scientific, medical or technological innovation that can change that.
Jennifer O’Connell is a feature writer and opinion columnist with The Irish Times
Commenting is now closed for this story.
FIRST Artificial Sperm EVER - YouTube
Could artificial sperm mean the end of men?
No men OR women needed: artificial sperm and eggs... | Daily Mail Online
Artificial sperm | The Sunday Times
Artificial Sperm | Request PDF
Latest Headlines
NASA
Apple
My Profile
Logout
Login
Wednesday, Feb 3rd 2021
12AM
-8°C
3AM
-8°C
5-Day Forecast
RELATED ARTICLES Previous 1 2 Next
Site
Web
Enter search term:
Search
Download our iPhone app
Download our Android app
Discover deals on home essentials and electricals
Apply AO.com voucher codes to save on home appliances
Check out the latest B&Q clearance for great offers
Save your pennies with eBay's deals
Keep yourselves entertained with these electrical offers
Check out the latest Wayfair sale to save on furniture
Home
News
U.S.
Sport
TV&Showbiz
Australia
Femail
Health
Science
Money
Video
Travel
DailyMailTV
Discounts
By Fiona Macrae for the Daily Mail Updated: 11:49 GMT, 29 October 2009
Human eggs and sperm have been grown in the laboratory in research which could change the face of parenthood.
It paves the way for a cure for infertility and could help those left sterile by cancer treatment to have children who are biologically their own.
But it raises a number of moral and ethical concerns. These include the possibility of children being born through entirely artificial means, and men and women being sidelined from the process of making babies.
Forever fertile? Infertile men and women could have their own biological children using the breakthrough sperm and eggs
Opponents argue that it is wrong to meddle with the building blocks of life and warn that the advances taking place to tackle infertility risk distorting and damaging relations between family members.
The U.S. government-funded research also offers the prospect of a 'miracle pill' which staves off the menopause, allowing women to wait longer to have a child.
It centres on stem cells, widely seen as a repair kit for the body.
Scientists at Stanford University in California found the right cocktail of chemicals and vitamins to coax the cells into becoming eggs and sperm.
Controversial: Britain's oldest mother Elizabeth Adeney, 67, who went abroad for IVF, is pictured here with her newborn son in June this year
The sperm had heads and short tails and are thought to have been mature enough to fertilise an egg.
The eggs were at a much earlier stage but were still much more developed than any created so far by other scientists.
The double success, published in the journal Nature, raises the prospect of men and women one day 'growing' their own sperm and eggs for use in IVF treatments.
The American team used stem cells taken from embryos in the first days of life but hope to repeat the process with slivers of skin.
The skin cells would first be exposed to a mixture which wound back their biological clocks to embryonic stem cell state, before being transformed into sperm or eggs.
Starting with a person's own skin would also mean the lab-grown sperm or eggs would not be rejected by the body.
The science also raises the possibility of 'male eggs' made from men's skin and 'female sperm' from women's skin.
This would allow gay couples to have children genetically their own, although many scientists are sceptical about whether it is possible to create sperm from female cells, which lack the male Y chromosome.
The U.S. breakthrough could unlock many of the secrets of egg and sperm production, leading to new drug treatments for infertility.
Defects in sperm and egg development are the biggest cause of infertility but, because many of the key stages occur in the womb, scientists have struggled to study the process in detail.
Researcher Rita Reijo Pera, of Stanford's Centre for Human Embryonic Stem Cell Research, believes new fertility drugs are just five years away.
However, safety and ethical concerns mean that artificial sperm and eggs are much further away from use.
Dr Reijo Pera said any future use of artificial eggs and sperm would have to be subject to guidelines.
'Whether one builds the boundaries on religion or just on an internal sense or of right and wrong, these are important. In this field, it is not "anything goes".'
Scientists at Newcastle University claimed to have made sperm from embryonic stem cells earlier this year but the research paper has been retracted.
Dr Allan Pacey, a Sheffield University expert in male fertility said: 'Ultimately this may help us find a cure for male infertility. Not necessarily by making sperm in the laboratory, I personally think that is unlikely, but by identifying new targets for drugs or genes that may stimulate sperm production to occur naturally.
'This is a long way off, but it is a laudable dream.'
Dr Peter Saunders, of the Christian Medical Fellowship, said that IVF should be the preserve of married couples.
'The question is, why are we creating artificial gametes (eggs and sperm) and aborting 200,000 babies a year when there are many, many couples willing to adopt?'
Should scientists be developing a 'miracle pill' so women can have children later in life?
Josephine Quintavalle, of the campaign group Comment on Reproductive Ethics, warned that any flaws in the artificial sperm or eggs could be passed on to future generations.
Anthony Ozimic, of the Society for the Protection of Unborn Children, said: 'The use of artificial gametes in reproduction would distort and damage relations between family members.
'There are no instances of any major medical advance achieved by abandoning basic ethical principles such as safeguarding the right to life.'
The comments below have been moderated in advance.
The views expressed in the contents above are those of our users and do not necessarily reflect the views of MailOnline.
We are no longer accepting comments on this article.
Published by Associated Newspapers Ltd
Part of the Daily Mail, The Mail on Sunday & Metro Media Group
Hard Big Black Cock Fucking
Outdoor 20
Femdom Pee Drink
Porno Granny Outdoor
Lana Rhoades Naughty America
















/%3Cimg%20src=)