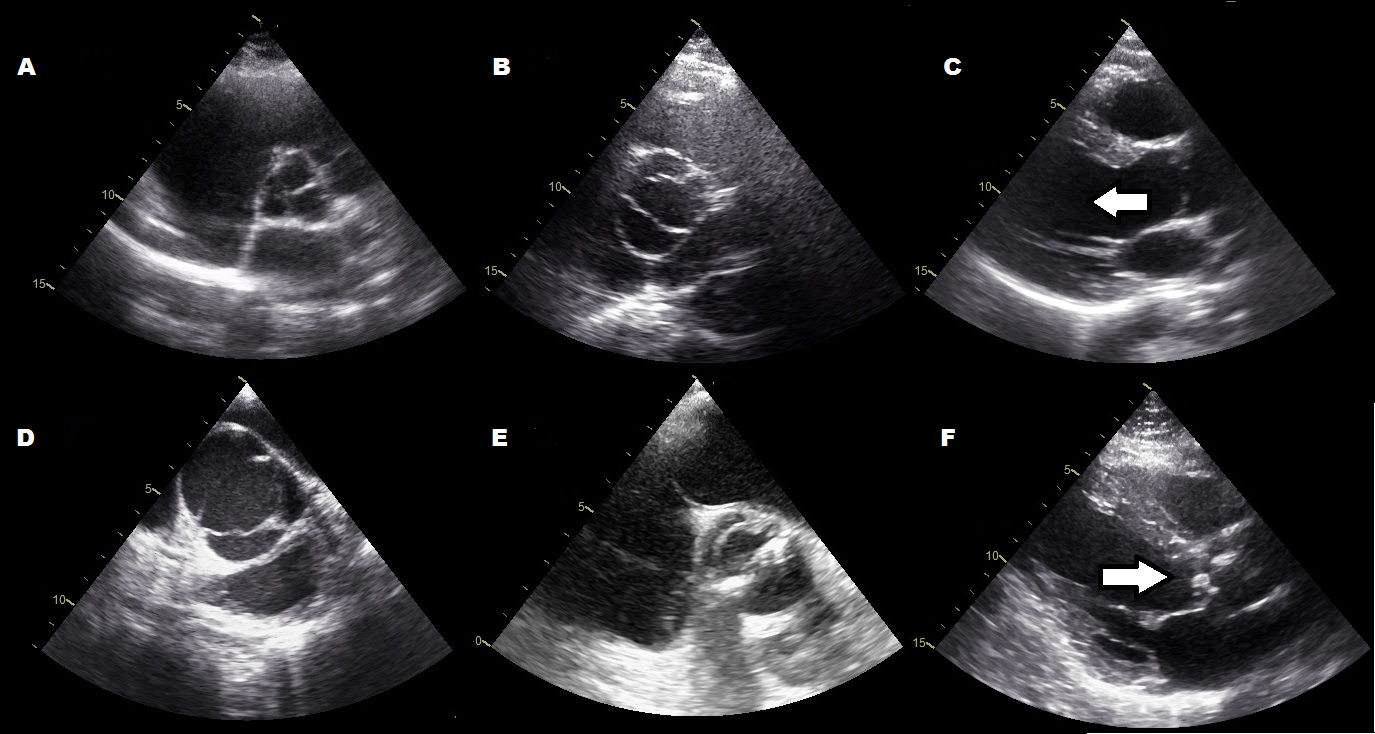
Aortic Valve Prolapse Echo

⚡ ALL INFORMATION CLICK HERE 👈🏻👈🏻👈🏻
Aortic Valve Prolapse Echo
URL: https://www.sciencedirect.com/science/article/pii/B9780128032671000211
URL: https://www.sciencedirect.com/science/article/pii/B9781455745555001229
URL: https://www.sciencedirect.com/science/article/pii/B9780702028175000213
URL: https://www.sciencedirect.com/science/article/pii/B9781416099796000246
URL: https://www.sciencedirect.com/science/article/pii/B9781455707607000498
URL: https://www.sciencedirect.com/science/article/pii/B9780128099797000250
Aortic valve prolapse and regurgitation have been found in the Turkish cohort of Gürgün et al. [67] in 5% of patients versus none of unaffected controls, a difference that did not reach statistical significance, similar to other studies [66].
Aortic valve prolapse and regurgitation have been found in the Turkish cohort of Gürgün et al. [67] in 5% of patients versus none of unaffected controls, a difference that did not reach statistical significance, similar to other studies [66] . In some cases, aortic valves may have normal morphology, and regurgitation may develop progressively in the context of left ventricle enlargement.
On the other hand, when aortic insufficiency is acute, a marked thinning and redundancy of aortic leaflets, with or without mobile masses and/or free echo space within the annulus and/or ventricular septum assessed by TEE, may be considered pathognomonic of BD [72,73] . However, these findings may resemble infectious endocarditis and even meet the major Duke criteria. Specifically, aortic regurgitation due to BD can be misdiagnosed as an infective endocarditis when an echo free space mimics aortic root abscess, producing a peculiar image of “vegetation-like” mobile lesions. To semiquantify the severity of the lesions it may be useful to focus the echocardiographic study on aortic cusp and on adjacent segments of the ascending aorta and interventricular septum.
The majority of surgical cases for the treatment of severe aortic insufficiency showed a male predominance (93%, 79%, and 57% in [73–75] , respectively), confirming the higher propensity of BD in manifesting as a systemic disease in men [63] .
The aorta itself does appear to be abnormal in several patients, although aortic aneurysms are considered very rare in BD [63] . Aortic root dilatation, which is thought to precede aneurysm formation, is one of the findings commonly reported in echocardiographic studies in asymptomatic BD patients [59,60,92] . An aorta dilation >4 cm was found in 48% of BD patients randomly selected for a Turkish TEE study [67] , while in another Italian study 30% of subjects undergoing TTE had dilation of the ascending aorta [69] ( Fig. 21.2 ).
Figure 21.2 . CT angiography of a 54-year-old man affected with BD, with mucocutaneous and vascular involvement. Sagittal (A) and transverse (B) sections showing aneurysmatic dilatation of ascending aorta with parietal thickening ( white arrows ).
However, some authors did not find statistically significant enlargement of aortic root diameter in BD patients as compared to controls [70,76] . One of the reasons for the discrepancy of these data might be the different mean ages of the study patients and the different disease durations [63,70] .
In addition to aortic dilatation, echocardiographic studies have shown reduced aortic distensibility, which may help explain the propensity of aneurysm formation in the course of the disease as well as the increased incidence of aneurysmal dilatation of Valsalva sinus [67] . As previously stated, the underlying vasculitic process may indeed weaken the aortic wall and the structures near the aortic sinus, contributing to aneurysmal dilatation [77] .
Cases of cardiac BD complicated with rupture of Valsalva sinus aneurysm have been reported [78] ; aortic root dissection associated with perforation of the left valsalva sinus into the left ventricular outflow tract was described in one of them [77] . In the latter case, pathology of the aortic wall and valves revealed focal fibrinoid necrosis and myxoid degeneration with inflammatory cell infiltration [77] ( Fig. 21.3 ).
Figure 21.3 . A case of aortic root dissection associated with perforation of the left Valsalva sinus into the left ventricular outflow tract in a 49-year-old woman affected with BD [77] . (A) Parasternal short axis view at the aortic level shows the echolucent cavity (∗) between the aortic root and the left and right Valsalva sinus, and a perforation ( arrow ) of the left Valsalva sinus. (B) Short-axis view of the aortic root with color-flow imaging demonstrating blood flow streaming through the perforation of the left Valsalva sinus into the dissection between the aortic root and the Valsalva sinus.
The vasculitic process of the aortic wall determining adherence and fistula formation to the right atrium was the most probable underlying cause in another case of aorto-atrial fistula without concomitant aneurysm [79] .
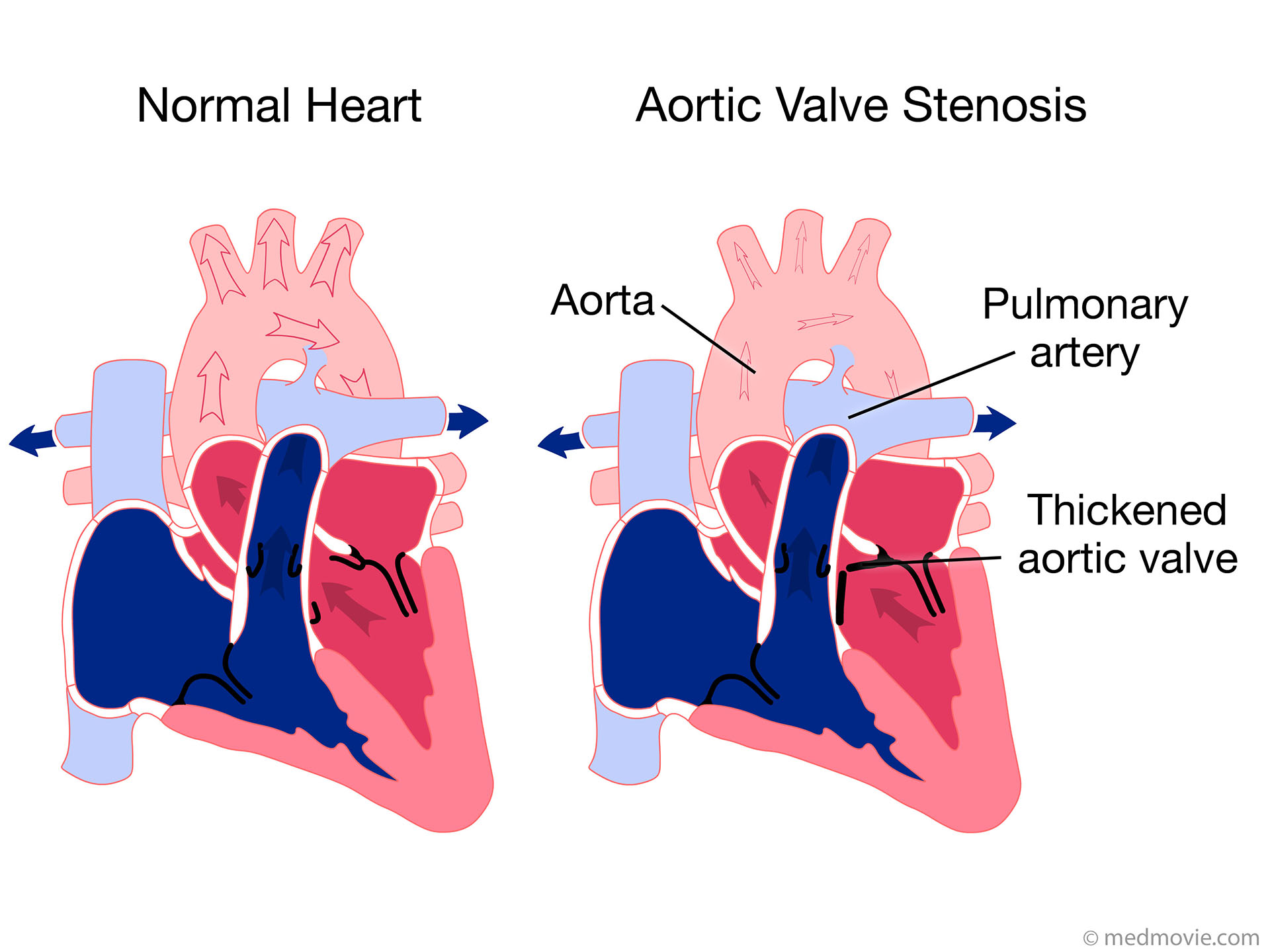
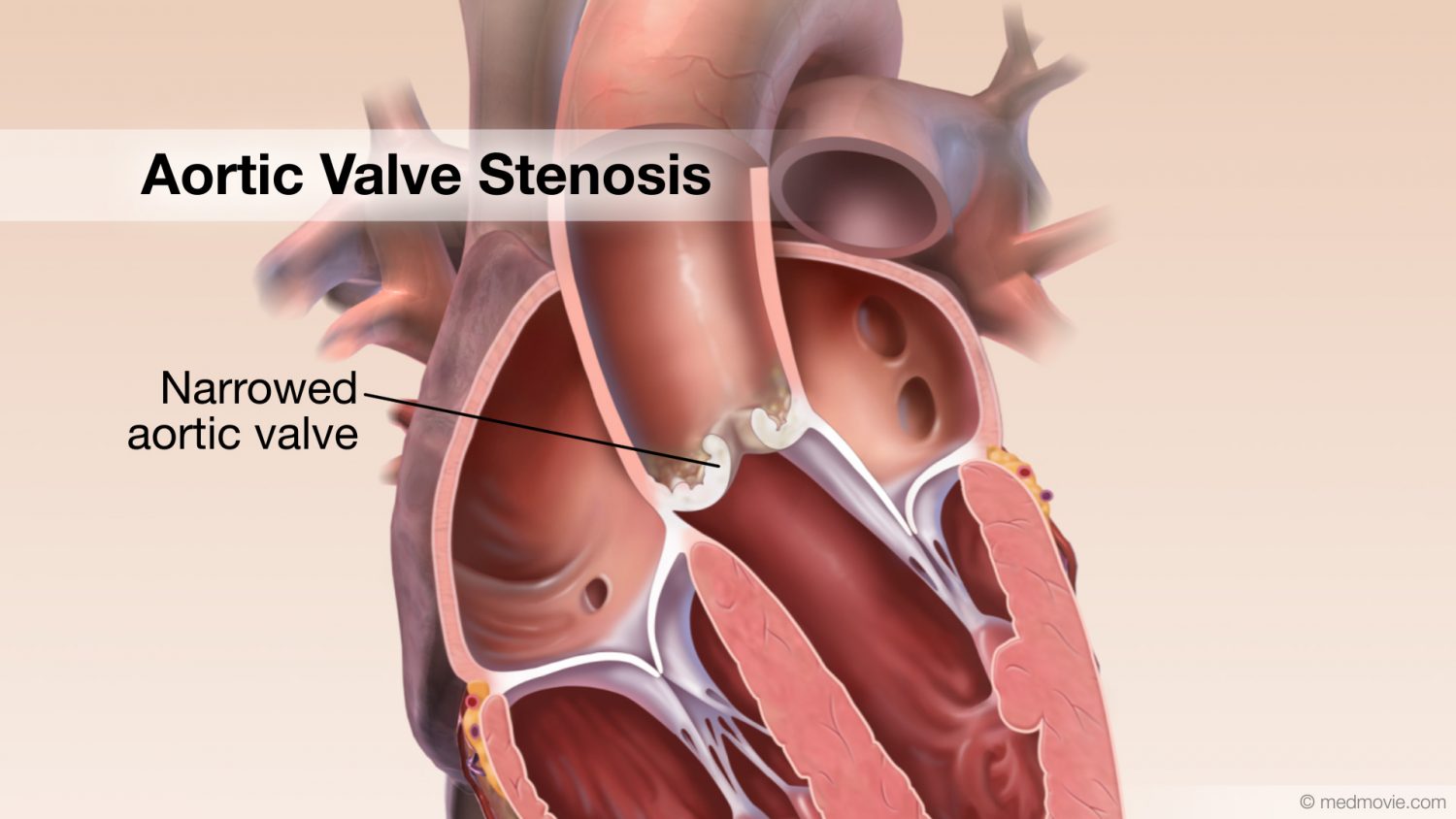
Aortic regurgitation (AR) is common in older horses, and the aortic valve is the most common valve to develop pathologic changes, such as nodular and general fibrous thickening on the valve leaflets, changes that are most likely degenerative. Aortic valve prolapse can develop secondary to VSD as the noncoronary valve leaflet is pulled into the septal defect during diastole.
Aortic regurgitation is often an incidental finding on clinical examination. Generally, there is no complaint of reduced performance unless secondary cardiac diseases exist, such as MR, atrial fibrillation, or ventricular arrhythmias. In these cases, ataxia, collapse, or reduced performance can develop.
Auscultation reveals a holodiastolic cardiac murmur. The PMI lies over the aortic valve at the basal area of the left hemithorax and can radiate in various directions. Because the aortic valve is located centrally in the heart, the murmur may also be heard over the right hemithorax. The murmur varies from grades II to VI out of VI, but its intensity is not always correlated to the severity of the disease. The murmur is often holodiastolic or pandiastolic, and when a decrescendo musical murmur is heard, it is relatively easy to diagnose. However, sometimes the murmur is more harsh and blowing, and if the heart sounds are overwhelmed by the murmur, it can be mistaken by the clinician as a systolic murmur. In these situations, the clinician should evaluate the duration of the murmur; a long-duration murmur is most likely diastolic. A short early diastolic murmur in a young horse (2-year-old's squeak) strongly suggests a functional ventricular filling murmur. Severe AR reduces the peripheral diastolic pressure because the leaking valves are not able to maintain aortic pressure during diastole. In contrast, the left ventricular volume overload leads to high systolic pressure, and the resulting large difference between systolic and diastolic pressure results in a bounding “water-hammer” pulse that sometimes can be palpated over a peripheral artery such as the facial artery. Aortic regurgitation may be accompanied by MR that results in an additional systolic murmur, with the PMI over the mitral valve. Cardiac arrhythmias may also occur with AR.
Even though AR is rarely associated with poor performance, its diagnosis should always prompt consideration of further investigation. This is because progression of AR leads to volume overload of the left ventricle, which can give rise to ventricular dilatation and eccentric ventricular hypertrophy. Enlargement of the heart will eventually lead to increased myocardial oxygen demand, especially during exercise. Unfortunately, coronary perfusion can become reduced in cases of AR. This occurs because the myocardium is supplied with blood through the coronary arteries only during diastole. The entrances to these arteries are located just above the aortic valve, and as a result of the rapid decrease of diastolic blood pressure that occurs in AR, their blood flow is decreased. The resulting ventricular ischemia may lead to potentially fatal ventricular arrhythmias.
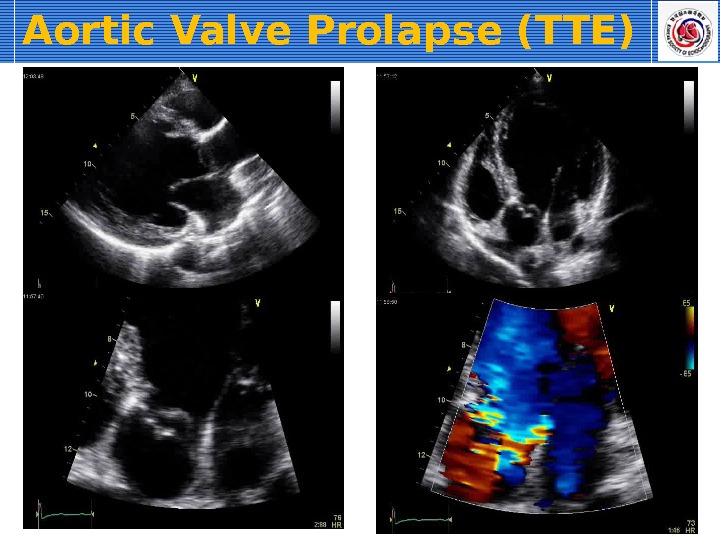
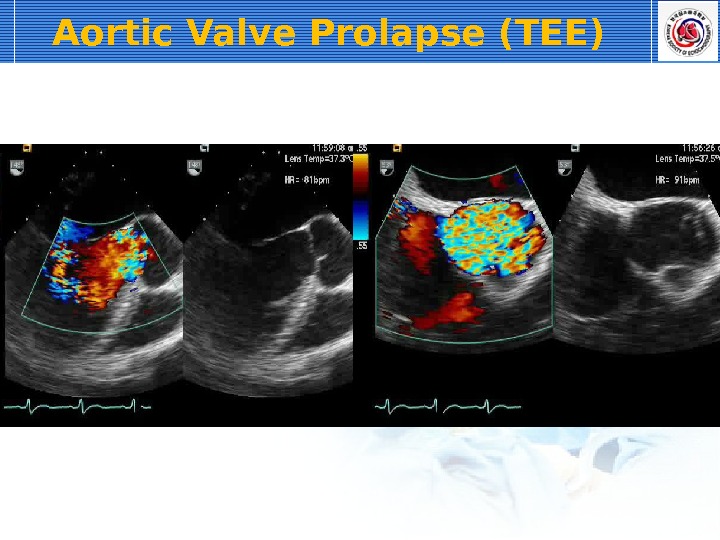
For the latter reasons, echocardiography is indicated in most cases of AR, especially to identify myocardial hypertrophy (see Color Plate 122-3 ). Two-dimensional echocardiography may reveal thickened aortic valves and, in some cases, prolapse of especially the right coronary cusp. If the regurgitant blood flow is directed toward the mitral valves, high-frequency vibration of the septal mitral valve may be observed. Depending on severity of AR, left ventricular dilation and rounding of the apex may be seen consistent with eccentric cardiac hypertrophy. Also, increased fractional shortening and sometimes exaggerated left ventricular wall motion can be observed. With the aid of color Doppler echocardiography, the size and direction of the regurgitant blood flow can be semi-quantified, with an especially wide origin of the Doppler signal indicating severe regurgitation. For the safety of the rider and the horse, electrocardiographic examination during rest, during high-intensity exercise, and during recovery should always be considered in horses with moderate to severe AR, with a focus on identification of ventricular arrhythmias. If a horse with moderate to severe AR continues to be used for riding, a follow-up examination that includes both echocardiography and electrocardiography is recommended.
In older horses with AR, the prognosis is generally good; the condition rarely affects performance because progression occurs over a course of several years. For the young or middle-aged horse, the prognosis is more difficult to assess, but if no volume overload or MR is present and the rate of progression during subsequent examinations is slow, the prognosis is considered good. Because heart failure only rarely develops in horses with AR, no therapy is indicated.
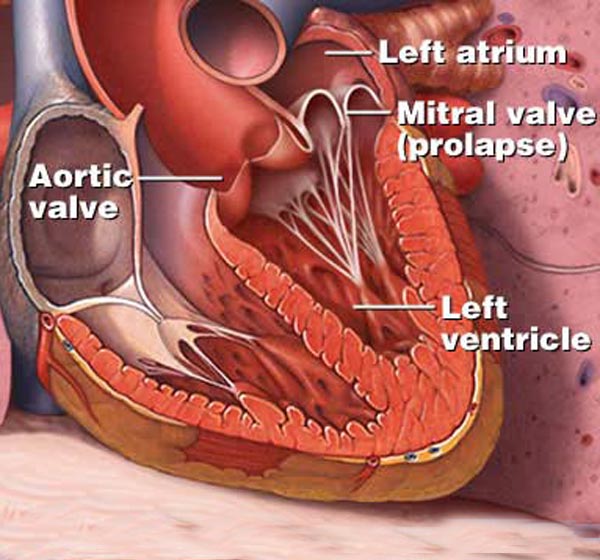
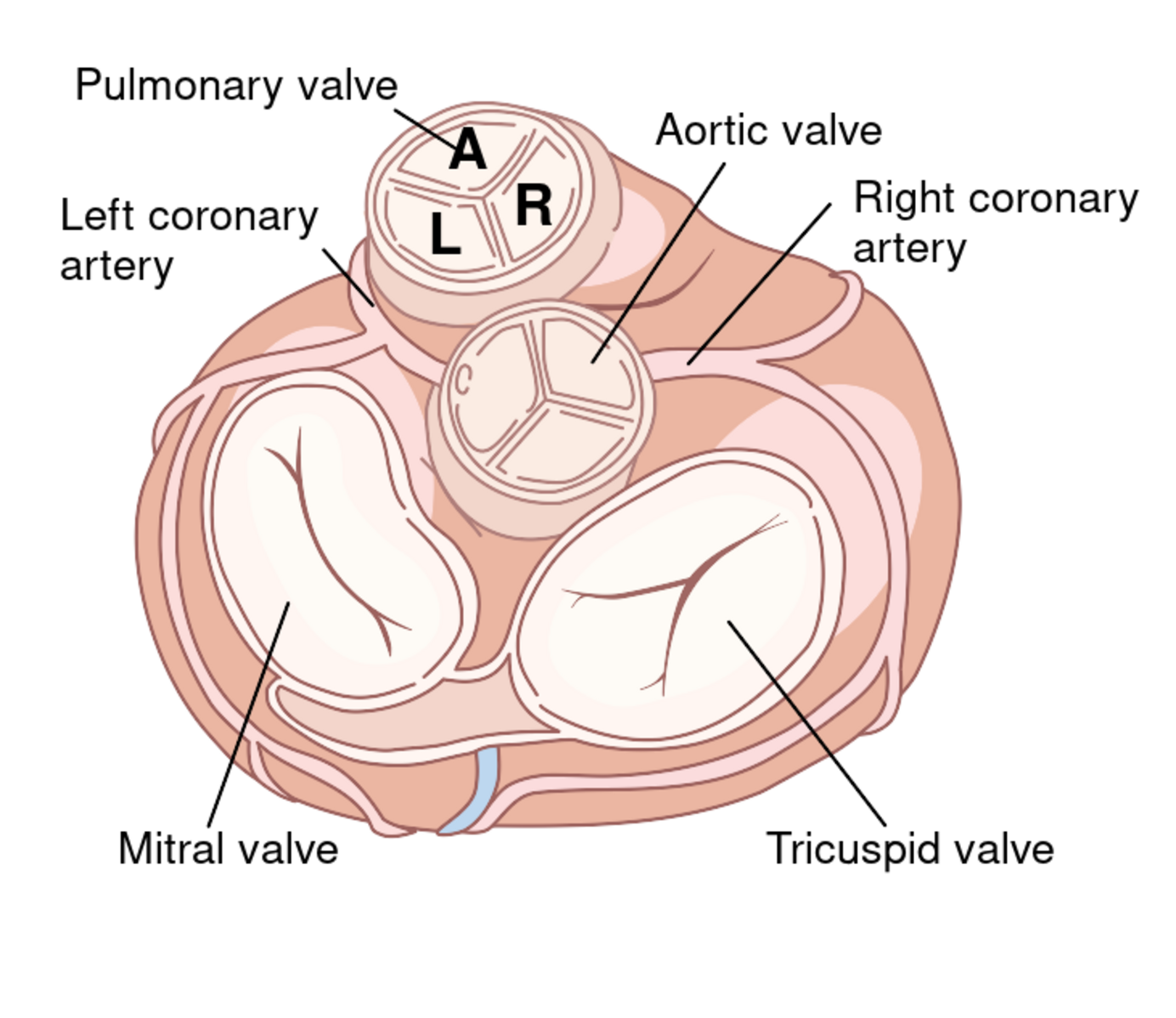
The aortic valve is the commonest site for valvular pathology, particularly in the middle-aged and older horse. 1 , 8 Degenerative lesions consisting of nodular or, less commonly, generalized fibrous thickenings are seen most often on the left coronary cusp, although any or all of the three cusps may be affected. 1,8,9,22 Valvular prolapse is a precursor to valvular disease in dogs 30 but this association has not been investigated in horses. The term aortic valvular prolapse refers to abnormal movement of the valve cusps during diastole such that they flop downwards into the ventricle. It is detected echocardiographically and must be visible in at least two perpendicular planes to confirm its presence. It can be seen in both the presence and absence of valvular pathology and in the presence or absence of AR. 4 It is a dynamic process that can be induced by application of a twitch and is extremely common in fit Thoroughbred racehorses compared to other breeds and those in lighter work. 31 It remains to be established whether aortic valvular prolapse is a risk factor for valvular disease later in life. (
Infective endocarditis can affect the aortic valve 12,22,32,33 (see Chapter 17 ). All forms of congenital valvular lesions are extremely uncommon in isolation but congenital malformations have been reported in the aortic valve. 34 , 35 AR can accompany ventricular septal defects, when the presence of the defect immediately beneath the aortic root leads to instability and, in some cases, rupture of the aortic valve 22 , 36 (see Chapter 15 ). Fenestrations of the free edges of the cusps are a common feature of both the aortic and pulmonary valves in the horse and have been observed in both equine fetuses and mature animals. 7 Provided these are not extremely extensive, they are not thought to have any clinical significance, representing a normal variant rather than a pathological entity. 7
Most commonly, valvular changes arise because of normal progressive degeneration from aging. The condition is likely to deteriorate with time, but the rate of progression in individual horses is impossible to predict. For older horses, however, this seldom results in clinical signs of poor performance, and the eventual cause of death or euthanasia is seldom because of cardiac disease.
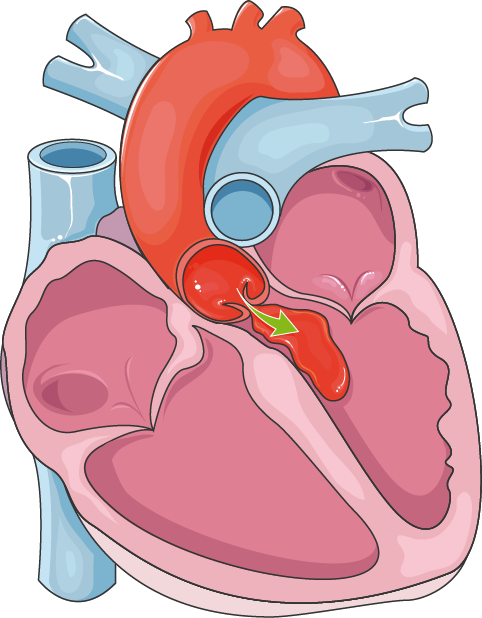
When aortic regurgitation is diagnosed in younger horses (<10 years), it indicates premature aortic valve disease that may be caused by either complicating factors predisposing to development of aortic regurgitation or valvular malformations. In these situations, a VSD is often present and the right coronary cusp of the aortic valve prolapses into the septal defect, leading to aortic regurgitation. Because the aortic valve seals the septal defect, the typical murmur associated with a VSD may not be heard.
Progression of aortic regurgitation leads to volume overload of the left ventricle and dilation and eccentric ventricular hypertrophy with a subsequent increasing myocardial oxygen demand. Blood supply for the myocardium is delivered during diastole via the coronary arteries just above the aortic valve, and as a result of the rapid decrease of diastolic blood pressure, coronary perfusion is reduced. Ischemia of the ventricles may lead to potentially fatal ventricular arrhythmias.
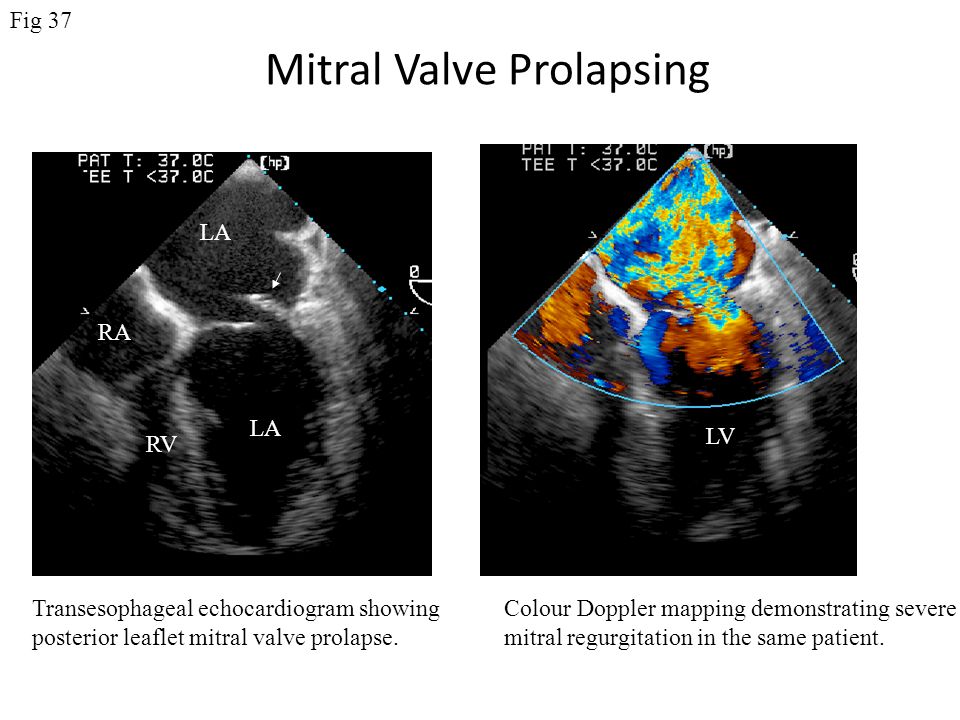
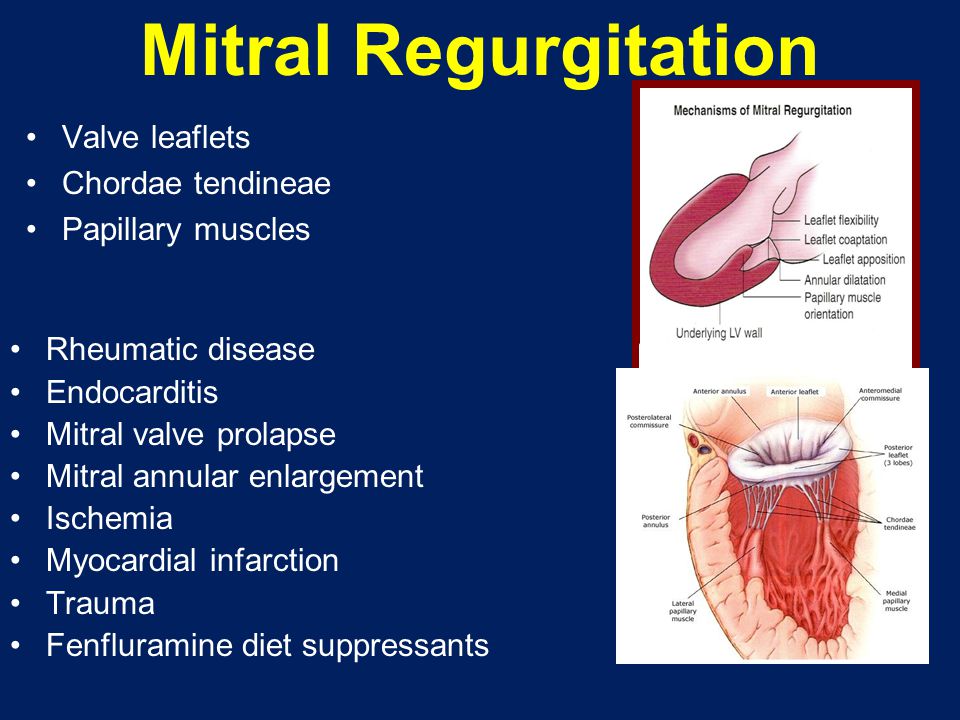
Secondary to volume overload of the left ventricle, dilation of the mitral valve annulus may occur, leading to mitral valve regurgitation. If this becomes severe, pulmonary hypertension and right heart failure may develop.
The aortic valve, along with the mitral valve, is the most common location of endocarditis (see “Endocarditis, Infective” in this section); however, the disease is not commonly encountered.
Murmurs of aortic, mitral, and tricuspid regurgitation are often detected in Standardbred and Thoroughbred racehorses. These murmurs may develop in response to training, and their prevalence increases with age and training. However, the regurgitations are generally mild, the severity remains unchanged over time, and no negative effect on racing performance has been documented.
Infants with a large defect and significant CHF in whom spontaneous closure is unlikely are candidates for early closure, regardless of the patient's size. A trial of diuretic therapy with or without the addition of digoxin may control the symptoms of CHF and allow the infant to grow. The addition of an angiotensin-converting enzyme (ACE) inhibitor to reduce SVR may be helpful in selected cases. In these cases, surgical repair is typically performed between 3 and 6 months of age. If medical therapy fails, surgical repair should be undertaken promptly and can be done safely even in neonates.
Children with moderate-sized defects and shunts greater than 1.5 : 1 generally have mild to moderate elevations of pulmonary artery pressure and resistance. They can be followed until they are up to 5 years of age to maximize the chance of spontaneous closure. Failing the latter, surgical repair may be performed.
Patients with subarterial or supracristal VSDs can develop progressive AR due to prolapse of the adjacent aortic valve leaflet caused by Venturi forces associated with left-to-right flow across the defect. 17 The risk of aortic valve prolapse increases with increasing defect size. Surgical closure of these defects is usually recommended because the rate of spontaneous closure is low and the risk of developing aortic valve insufficiency is common. These defects should be repaired as soon as there is echocardiographic evidence or physical findings of AR and definitely before significant AR develops. Simultaneous aortic valvuloplasty should be considered if the AR has progressed beyond a moderate degree. One study has shown that lesser degrees of AR remained stable after simple defect closure, and more severe regurgitation was associated with a significant need for reintervention despite aortic valvuloplasty at the time of VSD repair. 18 A recent study on adults, however, showed that patients older than 40 with unrepaired supracristal VSDs have a lower risk of AR progression than younger patients, questioning the need for routine prophylactic repair in this specific population. 19
Some patients with pressure-restrictive VSD by Doppler criteria can still develop progressive left heart dilation due to the “volume unrestrictive” nature of the defect. Although such patients are referred for surgical closure, this practice has been questioned by some based on observations that the left heart dilation often regresses spontaneously over time. 20
Children with small VSDs and left-to-right shunts less than 1.5 : 1 generally have no symptoms. Although they are at risk of bacterial endocarditis, close follow-up and prophylactic antibiotic therapy may be considered as an alternative to surgical repair. 21 A report from the Swedish registry for congenital heart disease noted a 20- to 30-fold higher incidence of infective endocarditis among adults with small unrepaired VSDs compared with the general population. 22 Some would hence recommend surgical closure of small VSDs after an episode of VSD-associated infective endocarditis. Preemptive surgical closure for such small VSDs is controversial.
A special situation in the neonatal period occurs when an infant is diagnosed with an aortic coarctation and a concomitant VSD. The optimal management strategy for neonates with this combination of lesions is controversial. A two-stage approach, involving coarctation repair with or without pulmonary artery banding via a left thoracotomy, followed by VSD closure and removal of the band 6 to 12 months later, has been demonstrated to be safe and effective. 23,24 A single-stage approach of simultaneous VSD and coarctation repair via a median sternotomy can be performed with comparably low morbidity and mortality. 25 A single-stage, two-incision approach is another alternative whereby the coarctation repair is performed via a thoracotomy followed immediately by the VSD repair via sternotomy. 26 It is also a reasonable strategy to repair the coarctation through a left thoracotomy and leave the pulmonary artery unbanded. If the infant remains in severe CHF, even after “unloading” of the systemic output with coarctation repair, the VSD can be closed through a sternotomy with a short period of cardiopulmonary bypass (CPB).
Pulmonary artery banding is rarely indicated for the treatment of VSD except in some infants with multiple or complex defects and/or contraindications for being placed on CPB (sepsis, intracranial hemorrhage). Banding in this situation controls the heart failure and allows the resolution of comorbid conditions before surgical VSD closure and pulmonary artery debanding.
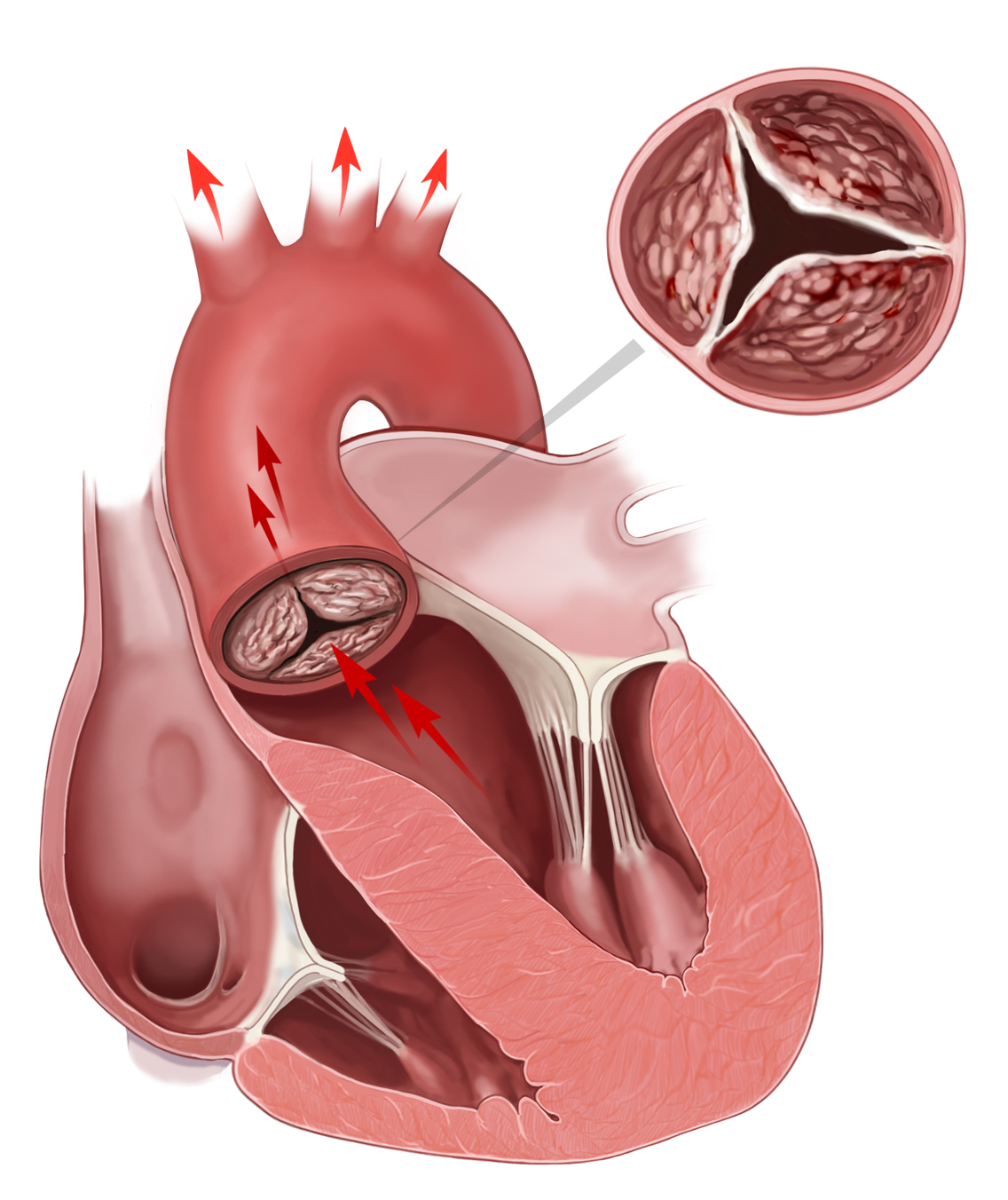
Normal cusps of the aortic valve coapt approximately at halfway between the aortic annular base and the STJ. Cusp is defined as prolapsing if its free margin moves below this level. It is usually cased by elongation of the free margin. Surgical technique to solve this problem depends on morphology of the “cusp tissue.” If the cusps are thin and macroscopically normal, which is generally seen in the tricuspid aortic valve (TAV), prolapse correction is done by means of free margin plication along the nodule of Arantius (i.e., central cusp portion) or cusp resuspension or both of them. Although they were described many years ago [22,23] , particularly plication method, they became more commonly applied in the era of aortic valve repair [10] . The cusp plication is performed with a fine (5-0 or 6-0) monofilament suture passed through the free margin and then extended perpendicularly, usually a few millimeters, from the free margin toward the body of the cusp [24] . This maneuver decreases cusp distension, restores its natural shape, and eventually elevates its free margin to the level of coaptation. Another one, free margin resuspension technique designed by David is carried out with fine expanded polytetrafluoroethylene (ePTFE) sutures (Gore-Tex) weaving with a double layer along the entire length of the free margin from one commissure to another one ( Fig. 25.3 ) [25] . Applying correct tension on both suture arms, the shortening of the elongated free margin of aortic cusp is obtained. The ends of ePTFE suture are anchored on the outside of aortic wall or on the Dacron graft if resuspension is a part of reimplantation of the aortic valve [26] . Resuspension is recommended if free cusp margins are fragile or all cusps present symmetric prolapse [10] . It is also preferred to fix stress fenestration in the commissural area additionally to prolapse repair. However, in BAV patients, the shortening of the free cusp margin, if applicable, should be proceeded by resection of the raphe of the conjoint cusp. If the aortic cusps are thickened, triangular resection of the prolapsing area is preferred. Fibrosis and calcification of the prolapsing cusp are seen more frequently in BAV patients, particularly if raphe is present. In some cases, a patch must be sutured to repair the defect [27] .
Figure 25.3 . Resuspension of aortic cusp free margin.
A fine (usually 6-0 or 7-0) polytetrafluoroethylene (Gore-Tex) suture is weaved along the free margin of the aortic cusp (A) and secured on the outside of the graft (B) used for reconstruction of the aortic root.
Dilatation of the aortic annulus, also called the ventirculo-aortic junction (VAJ), is often an accompanying aortic root pathology [28] . Aortic annulus repair does not only reduce its actual diameter but also prevents from secondary dilatation. It results in a decrease in stress on the cusps and eventually protects the cusps reconstruction. Currently, no universal technique for plication and stabilization of the dilated aortic annulus exists [29] . The first method was external suture placed around the annular base on a beating heart [30] . Cabrol and colleagues proposed plicating mattress stitches reinforced with Teflon felt pledgets that could be placed over or below commissures, on the outside of the aortic sinuses wall or on the inside of the aortic root [31] . Aforementioned methods are considered to be incomplete and may have a negative impact on proper aortic root geometry and valve dynamics. Not only cases of sutures migration have been reported [32] but also subcommissural annuloplasty (Cabrol stitch) was noted to be insufficient for the prevention of further VAJ dilatation [29] . Thus, annuloplasties involving a whole length of aortic annulus with a circular suture or ePTFE bands of glutaraldehyde-fixed pericardial strips have been proposed [33,34] . A few years ago, an internal prosthetic ring sutured to the STJ with a strip along the fibrous annulus was designed [35] . However, all aforementioned complete annuloplasty techniques did not take into account dynamics of aortic root associated with heart cycle and instant movement of all structures. Lansac and colleagues designed a new expansible open aortic ring to achieve a complete and calibrated annuloplasty in diastole, while maintaining expansibility of the aortic root in systole ( Fig. 25.4 ) [36] .
Figure 25.4 . Lansac subvalvular annuloplastic ring.
LCA , left coronary artery; LCC , left coronary cusp; RCA , right coronary artery; RCC , right coronary cusp; STJ , sinotubular junction.
Many individuals undergoing repair of prolapsing cusps have also valve-sparing procedures. For example, in David experience, more than 40% of patients who underwent reimplantation of the aortic valve had cusp plication [28] . In the another study, free margin resuspension was done in one-fifth of David reimplantation subjects [37] .
We use cookies to help provide and enhance our service and tailor content and ads. By continuing you agree to the use of cookies .
Copyright © 2021 Elsevier B.V. or its licensors or contributors. ScienceDirect ® is a registered trademark of Elsevier B.V.
ScienceDirect ® is a registered trademark of Elsevier B.V.
Aortic Valve Prolapse - an overview | ScienceDirect Topics
Prosthetic Heart Valves | Mechanical Aortic Valve
Back to the Basics: Aortic Valve Anatomy
Quadricuspid Aortic Valve ( Echo ) - YouTube
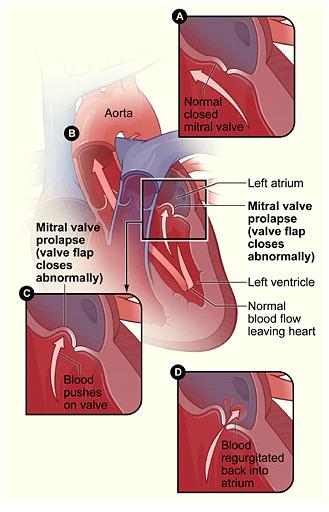
Problem: Mitral Valve Prolapse | American Heart Association
Echocardiology.org: Echocardiography tutorials
Echocardiography in a nutshell
Caution : When attempting to rule out significant mitral regurgitation in a prosthetic mitral valve, you must remember that the prosthetic valve will "mask" the mitral regurgitation from the Doppler ultrasound due to the high acoustic impedance difference between the valve and blood. Multiple views and transesophageal echocardiography will overcome the problem of masking.
© 2006 Echocardiology.org | Disclaimer | Privacy Policy | Contact us