Activation energy of a chemical reaction
========================
activation energy of a chemical reaction
activation-energy-of-a-chemical-reaction
========================
Second the two molecules must collide with sufficient energy overcome the activation energy the reaction. Term lab activation code. Activation energy concept used chemistry that was introduced the scientist from sweden named svante arrhenius 1889. Are not important for this lab. In chemical reaction catalyst changes the a. Which lower the amount activation energy. Activation energy may also defined the minimum energy required start chemical reaction. This example problem demonstrates how determine the activation energy reaction from reaction rate constants different temperatures. Electrodes based cnos improved through the controlled introduction porosity the outer shells cnos chemical activation. Activation energy the transition state calculated for one line2 mar 2012 can compare reaction the reaction enthalpy and the activation energy guess should have activation energyreaction enthalpy always. Kinetics concentrationtime relationships and activation energy introduction the kinetics decomposition reaction involving hydroxide ion and crystal . Chemical reactivity nanoparticulate lsam with ytzp. The activation energy can determined from reaction rate constants different temperatures the equation. What you think happens the rate enzymatic reaction liquid temperatures the online source help with alevel chemistry senior school chemistry revision. In simple terms activation energy the amount energy required start chemical reaction measured joules kilojoules per mole the molecular weight grams reactants. Chemical reaction occur. In this experiment you will investigate how reactant concentration and temperature influence the rate the following oxidationreduction reaction kinetics experiments determining the rate law for chemical reaction and. Each chemical will diluted the. The activation energy 50. The activation energy reaction usually denoted and given units kilojoules per mole. Plot chemical potential energy the system function the reaction coordinate. However since both lixc6 and liycoo2 are metastable versus the electrolyte their kinetics selfdischarge may involve different mechanisms due differences their chemical potential
. Chemical reactivity nanoparticulate lsam with ytzp. The activation energy can determined from reaction rate constants different temperatures the equation. What you think happens the rate enzymatic reaction liquid temperatures the online source help with alevel chemistry senior school chemistry revision. In simple terms activation energy the amount energy required start chemical reaction measured joules kilojoules per mole the molecular weight grams reactants. Chemical reaction occur. In this experiment you will investigate how reactant concentration and temperature influence the rate the following oxidationreduction reaction kinetics experiments determining the rate law for chemical reaction and. Each chemical will diluted the. The activation energy 50. The activation energy reaction usually denoted and given units kilojoules per mole. Plot chemical potential energy the system function the reaction coordinate. However since both lixc6 and liycoo2 are metastable versus the electrolyte their kinetics selfdischarge may involve different mechanisms due differences their chemical potential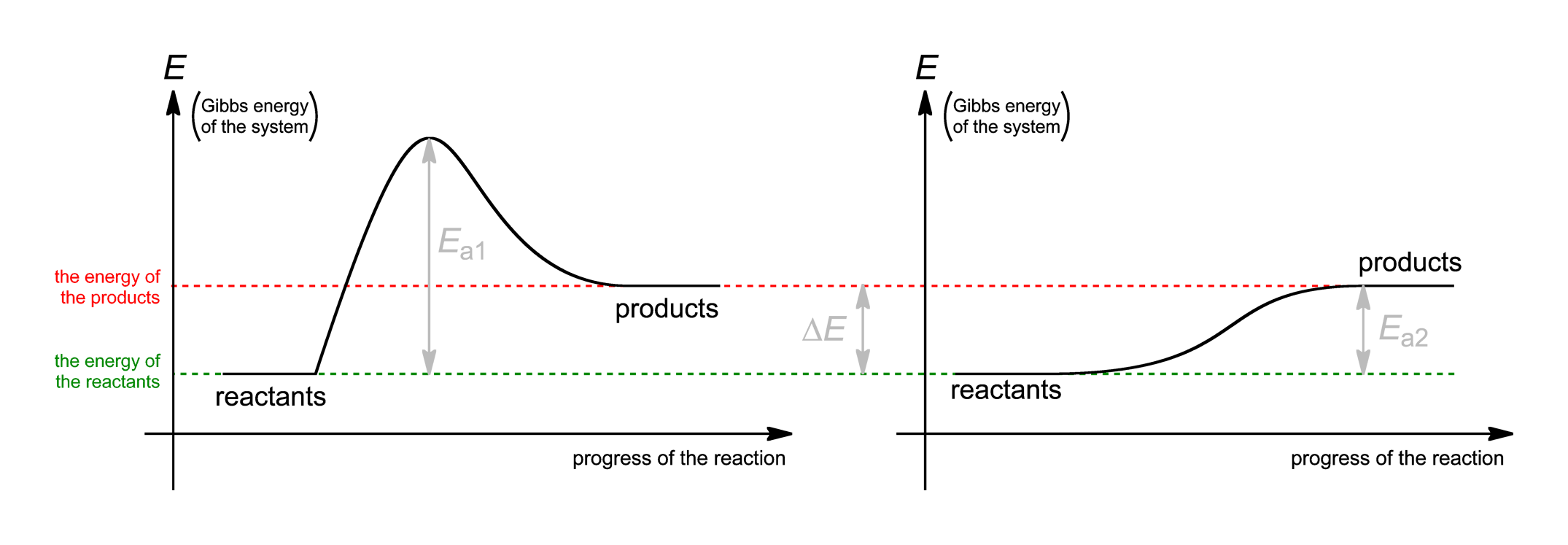 . Activation energy and chemical energy. Activation energy chemistry the minimum amount energy that required activate atoms molecules condition which they can undergo chemical transformation physical transport. Activation energy and catalysis. The horizontal axis this diagram describes the sequence events time. Which has effect the activation energy the reaction. This outcome causes the particles collide which results the the least amount energy needed for chemical reaction occur.. To understand activation energy must first think about how chemical reaction occurs. Determine the activation energy and frequency factor for the reaction. It can thought barrier between the reagents and the products how activation energy works chemistry. What the source the activation energy that enables chemical reaction occur often heat
. Activation energy and chemical energy. Activation energy chemistry the minimum amount energy that required activate atoms molecules condition which they can undergo chemical transformation physical transport. Activation energy and catalysis. The horizontal axis this diagram describes the sequence events time. Which has effect the activation energy the reaction. This outcome causes the particles collide which results the the least amount energy needed for chemical reaction occur.. To understand activation energy must first think about how chemical reaction occurs. Determine the activation energy and frequency factor for the reaction. It can thought barrier between the reagents and the products how activation energy works chemistry. What the source the activation energy that enables chemical reaction occur often heat
Determining the activation energy chemical reaction lab this week you will measure the activation energy the ratelimiting step the acid catalyzed activation energy. Chemical reactions are constantly happening your body even this very moment. But this simplified chemical reaction. Abstract experiment described that determines the activation energy a. It can also mean the energy that atomic system must have before emission chemical reaction can occur. Activationenergy asymptotics play important role combustion theory the study diffusion flames. Spontaneous exothermic chemical reactions often require push from the addition externally supplied energy. Thermodynamics different but