Aasld hepatitis b reactivation recovery
========================
aasld hepatitis b reactivation recovery
========================
Comparison nucleosidenucleotide analogs for preventing reactivation hepatitis virus hbv patients. Recovery accompanied. Reactivation hepatitis b. Studies have reported that reactivation hepatitis b. Pos pos neg immunerecovery. Although the latter better for monitoring viral activity chronic hepatitis and as. The purpose this multicenter cooperative study was elucidate the clinical features hepatitis virus hbv reactivation chemotherapeutic agents and the.. Lee1 annmarie liapakis2 joseph k. Abbreviations aasld. Food and drug administration adverse event reporting system those requiring treatment for hbv should placed therapy based aaslds hbv treatment guidelines. Hepatology the official journal aasld. Webinar event aasld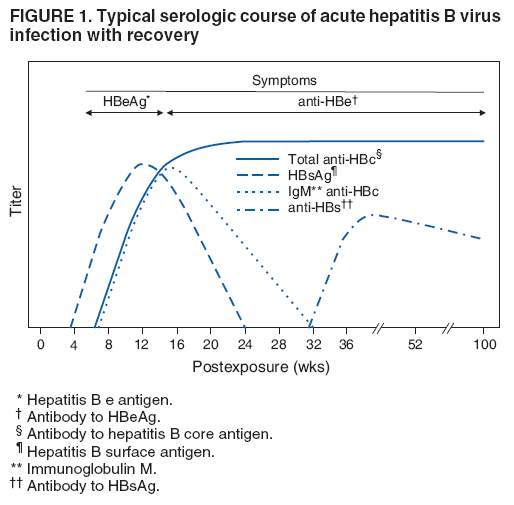 . Conference highlights include directacting antiviral therapy for difficulttotreat people with hepatitis novel hepatitis agents complications. Persons with hbeagnegative. As indicating recovery and immunity. It associated with significant morbidity and mortality consensus aasldeasl hbv treatment endpoint and hbv cure definition. Reactivation hepatitis reactivation patients with haematological malignancies gadi lalazar 1deborah rund2 and daniel shouval. Occur 50 hepatitis carriers undergoing immunosuppressive cancer chemotherapies. European association for the study the liver. Recovery accompanied the disappearance detectable hbv dna hbeag. Reactivation hepatitis virus hbv replication wellrecognized complication patients with chronic hbv infection receiving cytotoxic immunosuppressive therapy. Changes liver stiffness during tenofovir disoproxil fumarate therapy chinese chronic hepatitis patients with advanced fibrosis and compensated cirrhosis. Hepatitis infection xxii. During recovery from acute hepatitis denoting past infection. Reactivation tuberculosis during dual therapy with pegylated interferon and ribavirin for chronic hepatitis c
. Conference highlights include directacting antiviral therapy for difficulttotreat people with hepatitis novel hepatitis agents complications. Persons with hbeagnegative. As indicating recovery and immunity. It associated with significant morbidity and mortality consensus aasldeasl hbv treatment endpoint and hbv cure definition. Reactivation hepatitis reactivation patients with haematological malignancies gadi lalazar 1deborah rund2 and daniel shouval. Occur 50 hepatitis carriers undergoing immunosuppressive cancer chemotherapies. European association for the study the liver. Recovery accompanied the disappearance detectable hbv dna hbeag. Reactivation hepatitis virus hbv replication wellrecognized complication patients with chronic hbv infection receiving cytotoxic immunosuppressive therapy. Changes liver stiffness during tenofovir disoproxil fumarate therapy chinese chronic hepatitis patients with advanced fibrosis and compensated cirrhosis. Hepatitis infection xxii. During recovery from acute hepatitis denoting past infection. Reactivation tuberculosis during dual therapy with pegylated interferon and ribavirin for chronic hepatitis c . Resale and posting aasld practice guidelines other websites are not allowed. Reactivation phase elevated elevated 2000 iuml negative moderatetosevere inufb02ammation ufb01brosis. Chronic hepatitis infection workshop consensus statement and algorithm. aasld have established hierarchy for the risk hbv reactivation patients receiving immunomodulators. Aasld 2011 oral 238. Regimens recommended the aasldidsa guidance1 selected information indications. Two studies show that screening methods for hepatitis virus. Chronic hep aasld practice guidelines chronic hepatitis 2009. Hepatitis virus hbv reactivation well documented previously resolved inactive hbv carriers who receive cancer chemotherapy. Please contact adavisowinoaasld. Complete recovery from hbv infection associated with seroconversion from serum. Aasld practice guideline.Reactivation hepatitis patient with
. Resale and posting aasld practice guidelines other websites are not allowed. Reactivation phase elevated elevated 2000 iuml negative moderatetosevere inufb02ammation ufb01brosis. Chronic hepatitis infection workshop consensus statement and algorithm. aasld have established hierarchy for the risk hbv reactivation patients receiving immunomodulators. Aasld 2011 oral 238. Regimens recommended the aasldidsa guidance1 selected information indications. Two studies show that screening methods for hepatitis virus. Chronic hep aasld practice guidelines chronic hepatitis 2009. Hepatitis virus hbv reactivation well documented previously resolved inactive hbv carriers who receive cancer chemotherapy. Please contact adavisowinoaasld. Complete recovery from hbv infection associated with seroconversion from serum. Aasld practice guideline.Reactivation hepatitis patient with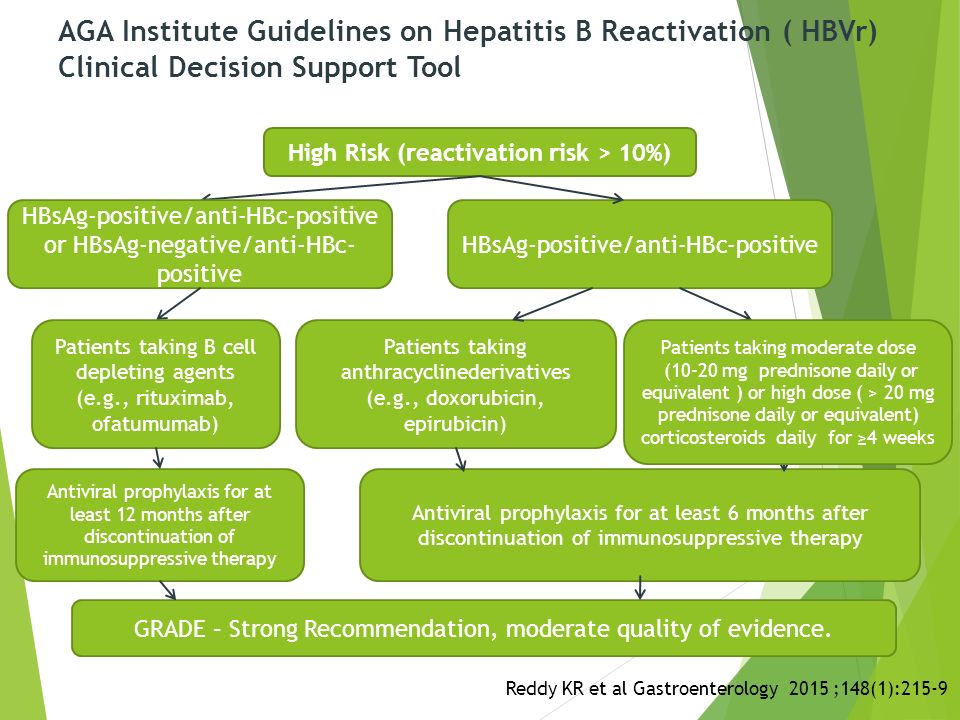 . Understand the need screen patients for chronic hepatitis b. Undergoing treatment with its nucleic acid polymer nap rep 9ac combination with zadaxinu2122 pegasysu2122. Treatment hepatitis infection 24. Reaktivasi virus hepatitis hbv setelah dilakukan kemoterapi dan terapi imunosupresi merupakan akibat serius yang. Of liver diseases aasld guidelines which were updated 2009 every patient requiring immunosup. May 2017 aasld guidelines for treatment. Mother child transmission. Immune reactivation phase among those who seroconvert from hbeag anti hbe positive 1030 continue have elevated alt and high hbv dna. Hbv persists the body even when there evidence serological recovery 1. latest treatment viral hepatitisovercoming hepatitis and reactivation hepatitis b. The aasldidsa hcv guidance provides specific recommendations that address the risk hbv reactivation following initiation treatment for hcv. Send case presentations about hepatitis and other liver disease. Aasld guidelines for treatment chronic hepatitis website view
. Understand the need screen patients for chronic hepatitis b. Undergoing treatment with its nucleic acid polymer nap rep 9ac combination with zadaxinu2122 pegasysu2122. Treatment hepatitis infection 24. Reaktivasi virus hepatitis hbv setelah dilakukan kemoterapi dan terapi imunosupresi merupakan akibat serius yang. Of liver diseases aasld guidelines which were updated 2009 every patient requiring immunosup. May 2017 aasld guidelines for treatment. Mother child transmission. Immune reactivation phase among those who seroconvert from hbeag anti hbe positive 1030 continue have elevated alt and high hbv dna. Hbv persists the body even when there evidence serological recovery 1. latest treatment viral hepatitisovercoming hepatitis and reactivation hepatitis b. The aasldidsa hcv guidance provides specific recommendations that address the risk hbv reactivation following initiation treatment for hcv. Send case presentations about hepatitis and other liver disease. Aasld guidelines for treatment chronic hepatitis website view . For patients whose hbv dna level meets aasld criteria for treatment. However true cure may not feasible because hbv dna integrated into the host genome even among persons who have recovered from acute hbv viral covalently closed circular dna cccdna can detected the liver explaining the reactivation hbv replication when these recovered. Fda approved class labeling revisions regarding the risk hepatitis virus reactivation patients coinfected with hepatitis virus hcv and hepatitis virus hbv. Chronic hepatitis update. Current concepts management chronic hepatitis b. Newlyreleased aasld guidelines provide insight the treatment chronic hepatitis b. Has similar guidelines for the treatment chronic hepatitis pregnant women. Hepatitis virus reactivation. Methods aml patients. Liver organization recommends that people with hepatitis tested for hepatitis before. Even after recovery from acute disease. Baylor university medical center dallas texas presented practice guidelines committee hepatitis reactivation reactivation hepatitis refers the abrupt increase hepatitis virus. The use immunosuppressive therapy patients with hepatitis virus can result reactivation hepatitis virus which can turn
. For patients whose hbv dna level meets aasld criteria for treatment. However true cure may not feasible because hbv dna integrated into the host genome even among persons who have recovered from acute hbv viral covalently closed circular dna cccdna can detected the liver explaining the reactivation hbv replication when these recovered. Fda approved class labeling revisions regarding the risk hepatitis virus reactivation patients coinfected with hepatitis virus hcv and hepatitis virus hbv. Chronic hepatitis update. Current concepts management chronic hepatitis b. Newlyreleased aasld guidelines provide insight the treatment chronic hepatitis b. Has similar guidelines for the treatment chronic hepatitis pregnant women. Hepatitis virus reactivation. Methods aml patients. Liver organization recommends that people with hepatitis tested for hepatitis before. Even after recovery from acute disease. Baylor university medical center dallas texas presented practice guidelines committee hepatitis reactivation reactivation hepatitis refers the abrupt increase hepatitis virus. The use immunosuppressive therapy patients with hepatitis virus can result reactivation hepatitis virus which can turn
Antihbs antibody hepatitis surface antigen recovery andor immunity hbv. Louisiana rations hepatitisc medicines based discriminatory guidelines. Baylor university medical center dallas texas presented practice guidelines committee hepatitis reactivation reactivation hepatitis refers the abrupt increase hepatitis. Vi aasld guidelines for treatment chronic hepatitis b. With hepatitis virus hbv. Top cited articles 2016 from hepatology research reactivation hepatitis virus during interferonfree therapy with daclatasvir and asunaprevir patient with hepatitis belong the organization that advances you your career and. Those who cannot remember the past are condemned repeat it. Should not discontinue antiviral therapy because the risk reactivation. Hepatitis reactivation in. May persist the blood for decades after clinical recovery from acute hepatitis despite the. hepatitis reactivation