12 Different Ways to Organize the Periodic Table of Elements
 The Periodic Tables You’ve Never Seen Before
The Periodic Tables You’ve Never Seen BeforeThe Periodic Table of Elements is an iconic image in classrooms and laboratories all around the world.
Yet despite having an almost unanimous agreement amongst scientists on its composition, there are over 1,000 different periodic tables—and that number continues to grow. This is because the standard table does not highlight all of the existing relationships between the elements.
With 118 elements currently known, there are many different interactions and stories to tell. Here are some of the most remarkable, fascinating and bizarre periodic tables that we could find.
Purpose and Properties of the Periodic TableTo understand why there are so many periodic tables, we first need to understand exactly what a periodic table is. “Periodic” tables get their name because they organize the chemical elements by periodicity (or recurring periodic patterns).
When the elements are organized by their relatively stable number of protons, for example, we get the standard periodic table of elements first devised by Russian chemist Dmitri Mendeleev in the year 1869.
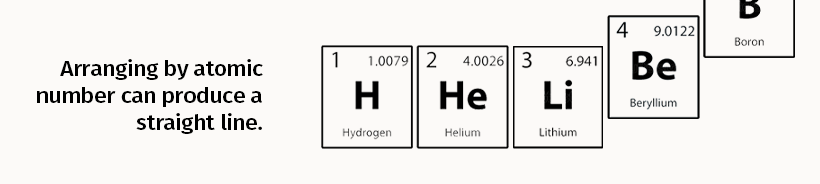
Compared to protons, electrons are very mobile. The way electrons are configured around a nucleus—and how they behave with the electrons of other elements—gives them specific chemical properties.
These properties are similar amongst some elements and repeat periodically. The standard periodic table of elements visualizes this by arranging elements based on these shared chemical properties, providing a guide for understanding similar electron configurations at a glance.
Examining Different Periodic TablesBelow are just a few of the most interesting and unique periodic table permutations not commonly used.
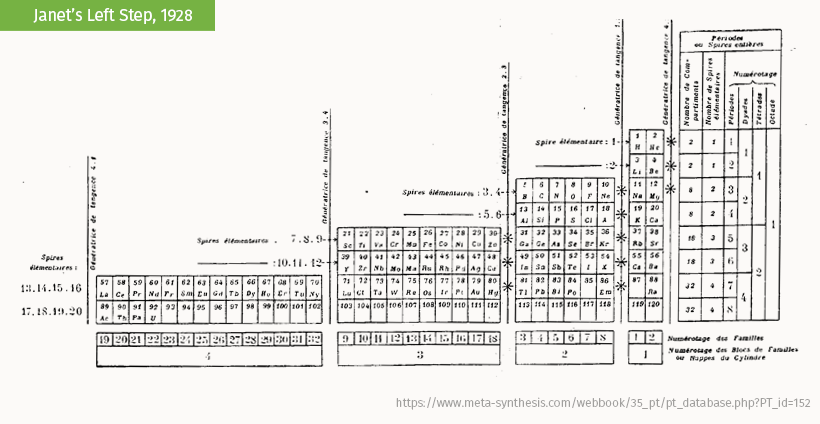
Charles Janet’s “left-step” periodic table is the most popular alternative table. It organizes the elements by the way that electrons fill orbitals (the regions they whir in) rather than valence (an electron’s ability to bond).
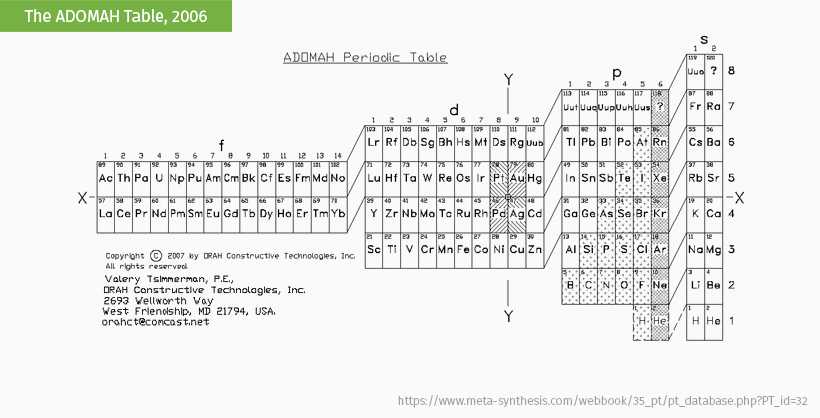
The ADOMAH table by Valery Tsimmerman is a form of the left-step table that groups elements by their principal quantum number.
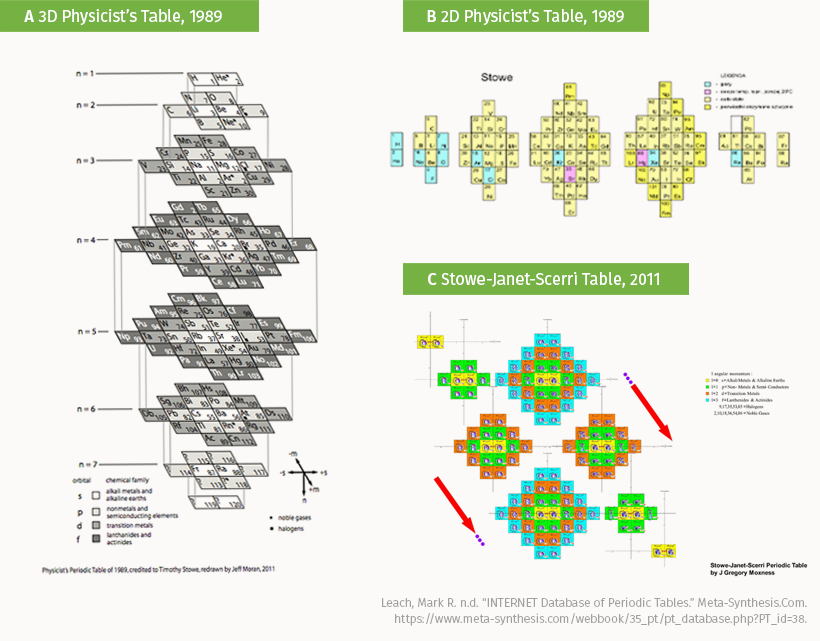
The Physicist’s Periodic Table of Elements by Timothy Stowe rearranges the standard table into both 3D-vertical (A) and 2D-horizontal (B) layouts. When merged with the work of Charles Janet and Eric Scerri (C) the result is a unique map of chemical groups.
Many researchers and scholars also continue to come up with new ways to show the basics of chemical periodicity.
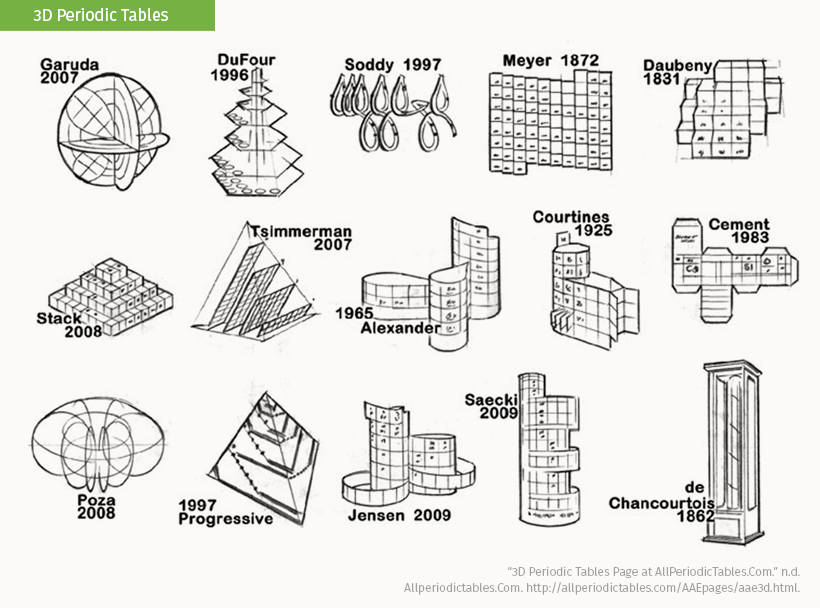
One method is in three-dimensional interpretations of periodicity. Here’s a collection of some of the most well-known designs and their creators, including the Telluric Screw and the Alexander Arrangement from the main graphic above.
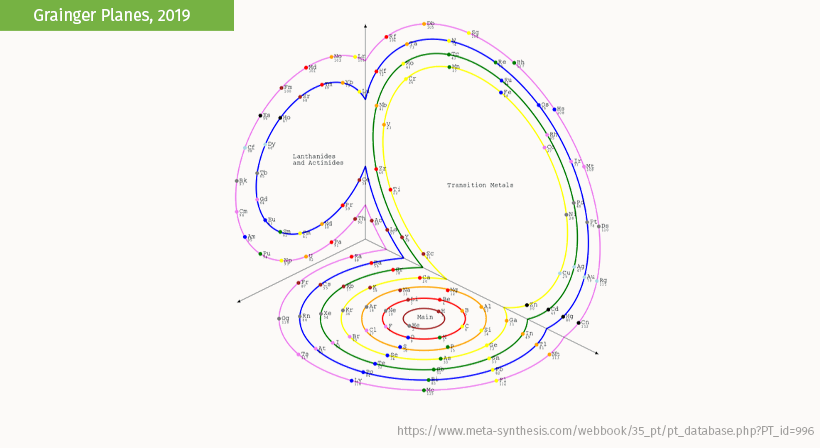
This representation by Anthony Grainger imagines all periods aligning along orthogonal (right-angle) planes cutting a sphere.
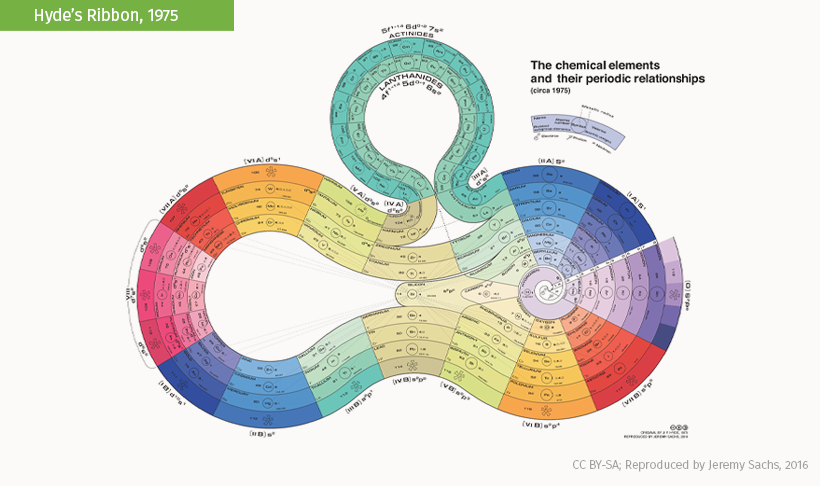
This eye-catching arrangement by Franklin J. Hyde winds a linear count of the elements while putting silicon at center stage.
Not all periodic tables show periodicity like the standard periodic table. Depending on the message, the chemical elements can take on various unique and exciting ways to tell a different kind of story.
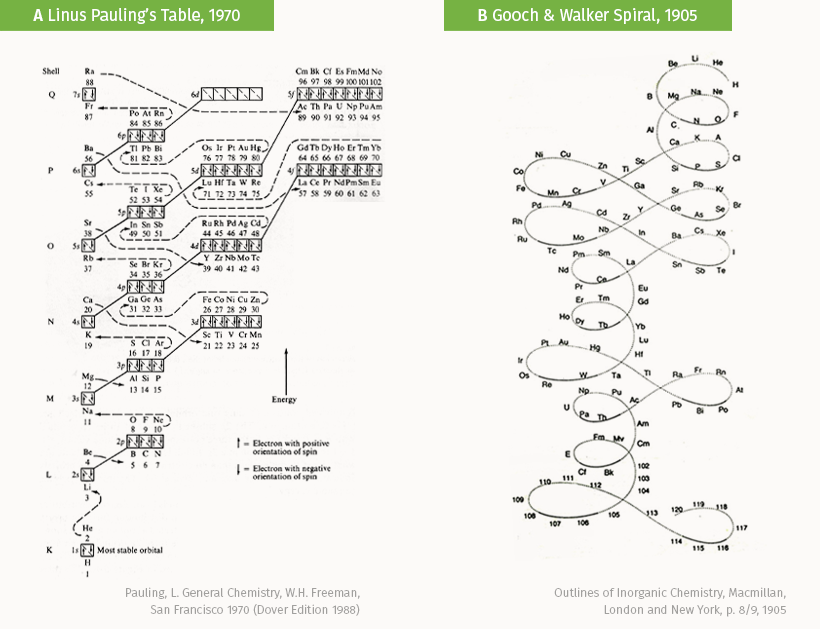
This flowchart from Linus Pauling’s “General Chemistry” (A) organizes elements by the energy levels of their electron shells and subshells. On the other hand, Gooch & Walker’s Spiral (B) is a figure-8 representation that is almost entirely devoid of most atomic information common to other periodic tables.
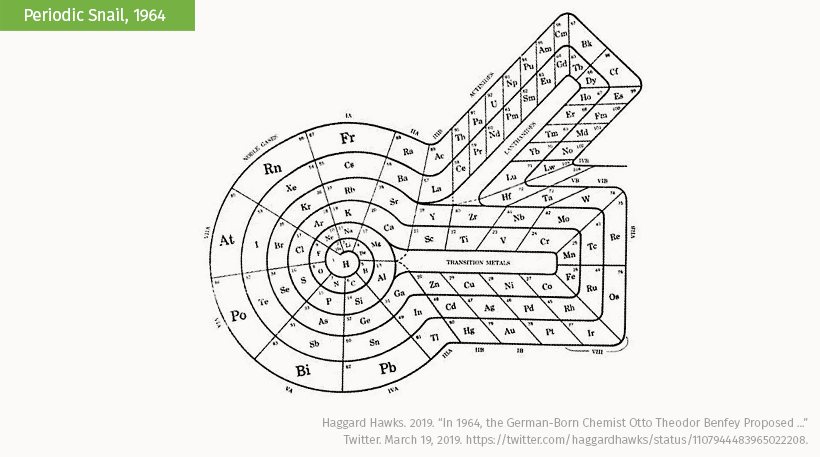
The spiral periodic table known as the Periodic Snail by Theodor Benfey swirls outwards by atomic number before branching into additional groups. The unique aspect of this table is that it reserves space for a hypothesized family of elements, the superactinides, and how they might correlate with the rest of the elements.
These are just a few of the hundreds and thousands of different ways to examine the elements.
As contemporary research continues to break new ground in our understanding of the elements, the opportunities to see the periodic table take on more new forms are shaping an exciting future for chemistry lovers and and data visualizers alike.
For more variations and designs of the periodic table, please visit Dr. Mark R. Leach’s online database at The Chemogenesis Web Book.
The post 12 Different Ways to Organize the Periodic Table of Elements appeared first on Visual Capitalist.