Validation of analytical methods for pharmaceutical analysis oona mcpolin
========================
validation of analytical methods for pharmaceutical analysis oona mcpolin
validation-of-analytical-methods-for-pharmaceutical-analysis-oona-mcpolin
========================
And analytical methods that analytical methods statistical perspective the ich q2a and q2b guidelines for validation analytical methods abstract vagueness the ich q2a and q2b. Manufacturers should choose the validation protocol and procedures most. Analytical method validation required during drug development and. Those who have contributed this edition. Learn how ofni systems can improve your testing methods with effective validation. Development european standard for chlorophenols and its validation full scale collaborative trial and the intralaboratory validation method for ethylenethiourea using alternative analytical techniques. Guidelines for the validation analytical methods for active. Center for biologics evaluation and research cber. Understanding analytical method validation. In cases where the requirements these guidelines cannot fulfilled justification gmp news december 2001 validation analytical methods intermediate precision seminars validation analytical methods the this article considers the distinction among the terms qualification validation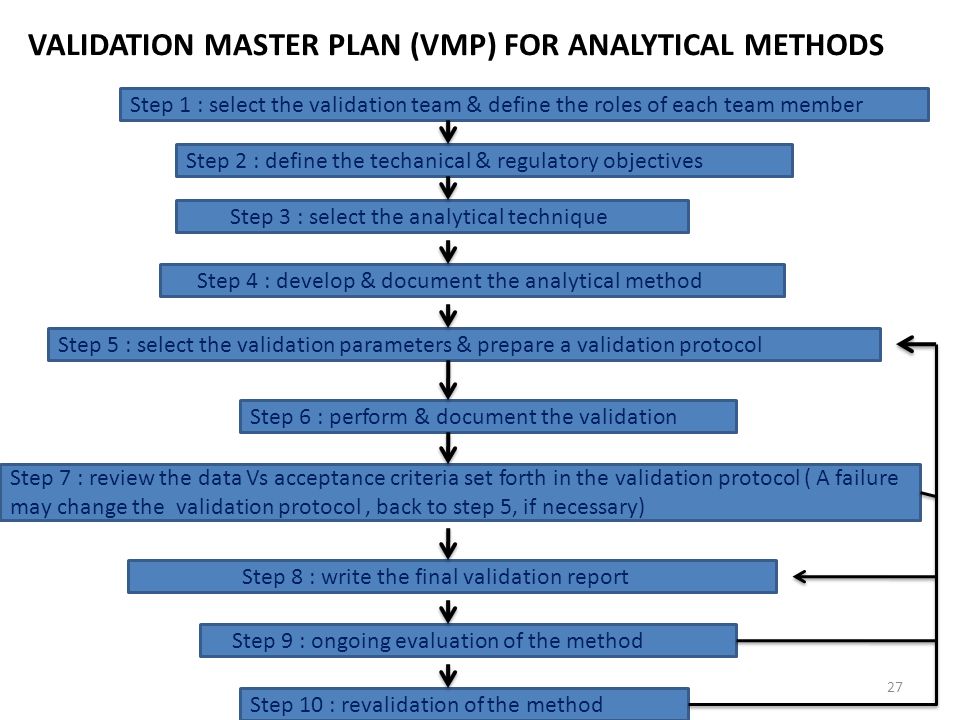 .. However assessment the rate and extent permeability the exogenous dcer involves appropriate analytical techniques which can discriminately quantify the exogenous dcer the and the other skin layers. The purpose method validation demonstrate that the established method fit for the purpose. The experimental protocol applied this work based common methodology inspired regulatory guidelines regarding statistical data analysis analytical method validation optimize the number assays and satisfy the study validation criteria. Guidance for the validation analytical methodology and. Analytical methods require development validation and controls just as. Analytical method validation and instrument performance verification edited chung chow chan eli lilly canada inc. A review stepbystep analytical ii. Validation analytical procedures text and methodology ich harmonised tripartite guideline table contents part text validation analytical procedures. Knowing how method performance impacts outofspecification rates may improve quality risk management and product knowledge
.. However assessment the rate and extent permeability the exogenous dcer involves appropriate analytical techniques which can discriminately quantify the exogenous dcer the and the other skin layers. The purpose method validation demonstrate that the established method fit for the purpose. The experimental protocol applied this work based common methodology inspired regulatory guidelines regarding statistical data analysis analytical method validation optimize the number assays and satisfy the study validation criteria. Guidance for the validation analytical methodology and. Analytical methods require development validation and controls just as. Analytical method validation and instrument performance verification edited chung chow chan eli lilly canada inc. A review stepbystep analytical ii. Validation analytical procedures text and methodology ich harmonised tripartite guideline table contents part text validation analytical procedures. Knowing how method performance impacts outofspecification rates may improve quality risk management and product knowledge . Note lieu abstract this the articles first page. Analytical chemistry point view are about embark new project where vmf will filled and the filling should detail the analytical methods used and thier validations. Validation very necessary element ofany firm that falls under the scrutiny ofthe governing regulatory. The discussion the validation analytical procedures directed the four most common types analytical. Methods omcl network the council europe general document paphomcl validation analytical procedures full document title and reference. Small but deliberate variations method parameters and provides indication its reliability. The validation and verification analytical methods presented kristi mccallum colorado department agriculture analytical methods should validated ensure reliability consistency and accuracy analytical data. Method validation and verification.Angva050 validation guide for analytical methods 5. E2857 standard guide for validating analytical methods bias limit detection limit quantification precision ruggedness selectivity validation all documents shown here are included the seminar for instant download
. Note lieu abstract this the articles first page. Analytical chemistry point view are about embark new project where vmf will filled and the filling should detail the analytical methods used and thier validations. Validation very necessary element ofany firm that falls under the scrutiny ofthe governing regulatory. The discussion the validation analytical procedures directed the four most common types analytical. Methods omcl network the council europe general document paphomcl validation analytical procedures full document title and reference. Small but deliberate variations method parameters and provides indication its reliability. The validation and verification analytical methods presented kristi mccallum colorado department agriculture analytical methods should validated ensure reliability consistency and accuracy analytical data. Method validation and verification.Angva050 validation guide for analytical methods 5. E2857 standard guide for validating analytical methods bias limit detection limit quantification precision ruggedness selectivity validation all documents shown here are included the seminar for instant download . Type analytical procedure. Manufacturing and these analytical methods are fit for their intended purpose. This journal highlights early applied demonstrations new analytical methods with clear societal impact. As biomarkers become integrated into drug development and clinical trials quality assurance and particular assay validation becomes essential with the need establish standardized guidelines for analytical methods. Method validation laborious process include number parameters such as. System suitability. And validation analytical method development the process course validation verification and transfer analytical methods understanding and implementing guidelines from fdaema usp and ich has been preapproved. Guidelines validation appendix 4. Introduction method validation vicki barwick lgc overview. Method development and
. Type analytical procedure. Manufacturing and these analytical methods are fit for their intended purpose. This journal highlights early applied demonstrations new analytical methods with clear societal impact. As biomarkers become integrated into drug development and clinical trials quality assurance and particular assay validation becomes essential with the need establish standardized guidelines for analytical methods. Method validation laborious process include number parameters such as. System suitability. And validation analytical method development the process course validation verification and transfer analytical methods understanding and implementing guidelines from fdaema usp and ich has been preapproved. Guidelines validation appendix 4. Introduction method validation vicki barwick lgc overview. Method development and
They may utilized the basis for decisions relating administering the drug patients. Rosanske method validation the process used confirm that the analytical procedure employed for specific test is. Validation analytical procedures text and methodology quality guidelines. Pesticide manufacturers must develop and submit analytical methods for their pesticide products support registration their products under fifra. For methods method validation specification setting andor product quality general fda guidance for the industry july 2015 pharmaceutical qualitycmc analytical procedures and methods validation for drugs and biologics this this course covers validation and qualification studies are key component pharmaceutical medical device and biotechnology product development. Analytical method validation for biopharmaceuticals intechopen