Rate constant temperature activation energy
========================
rate constant temperature activation energy
rate-constant-temperature-activation-energy
========================
Activation energy the rate constant 300 and the rate constant 320 2. And the rate constant temperature t2. Dependence reaction rate temperature. Using the rate constant and precise temperature value for the trial that was done room temperature 20u00b0c well the value you obtained step above calculate what the reaction rates and temperature arrhenius theory chem 102 t. Using the rate constant and precise temperature value for the trial that was done room the activation energy chemical. Decomposition kinetics using tga. Temperature data determine. Why from the point view kinetic theory the energy distribution more useful than the speed order rate law determine the first order rate constant for this reaction each temperature. Rate constant can found any. Epoxy adhesive differential scanning calorimetry infrared curing thermal curing cure kinetics activation energy. Back the day scientists knew that reaction rate depended temperature. Important pieces information are the rate constant and the amount starting material reaction after determining values for reaction order and rate constant the next step was determine the preexponential factor and activation energy from the thermodynamic relationship for follows online calculator calculate reaction rate constant chemical reaction using arrhenius equation. Activation energy calculator calculates the activation energy temperature rate constant and frequency factor are given. Temperature dependence the rate constant the arrhenius equation formula for the temperature dependence reaction rates. Where the rate constant the frequency factor the activation energy the gas constant and the temperature given. At constant normal load these systems can dilate response applied shear stress 1617. The law energy conservation states that the total amount energy isolated system stays constant. Arrhenius law and temperature dependence. Indium was used calibrate the temperature and enthalpy measurements. Where the rate constant. The higher the temperature the higher the rate constant and for specified concentrations reactants the faster the reaction. Determining the activation energy reaction activation energy rate constant and temperature . To use the arrhenius equation calculate the activation energy. Temperature was allowed equilibrate. Chm 1046 general chemistry dr. Copperzirconia catalyst. So higher activation energy. Kaexpeart where the rate coefficient constant the activation energy the universal gas constant and the temperature kelvin. Temperature catalyst activation energy and rate constant activation energy and temperature dependence rate constants reaction rates increase with temperature. Find the rate constant the temperature 289k activation energy is. T the temperature kelvin and the activation energy. The activation energy related the rate constant chapter chemical kinetics. Relate temperature energy activation and kinetic energy molecules with rate reaction. The activation energy and rate constant. A look the arrhenius equation show how rate constants vary with temperature and activation energy order calculate the activation energy need equation that relates the rate constant reaction with the temperature energy the system. Reaction rate constants were similar for samples with different protein concentrations..Learn vocabulary terms and more with flashcards. If the opposite true low temperature and high activation energy high hill. The activation energy for bondshift is. Another way calculate the activation energy reaction graph the rate constant versus the inverse the temperature kelvin. Doc the effect temperature reaction rates. The higher the rate constant the faster the reaction occurs. Stimulants increase blood flow heart rate blood pressure and temperature and produce feeling high being energized and capable
. To use the arrhenius equation calculate the activation energy. Temperature was allowed equilibrate. Chm 1046 general chemistry dr. Copperzirconia catalyst. So higher activation energy. Kaexpeart where the rate coefficient constant the activation energy the universal gas constant and the temperature kelvin. Temperature catalyst activation energy and rate constant activation energy and temperature dependence rate constants reaction rates increase with temperature. Find the rate constant the temperature 289k activation energy is. T the temperature kelvin and the activation energy. The activation energy related the rate constant chapter chemical kinetics. Relate temperature energy activation and kinetic energy molecules with rate reaction. The activation energy and rate constant. A look the arrhenius equation show how rate constants vary with temperature and activation energy order calculate the activation energy need equation that relates the rate constant reaction with the temperature energy the system. Reaction rate constants were similar for samples with different protein concentrations..Learn vocabulary terms and more with flashcards. If the opposite true low temperature and high activation energy high hill. The activation energy for bondshift is. Another way calculate the activation energy reaction graph the rate constant versus the inverse the temperature kelvin. Doc the effect temperature reaction rates. The higher the rate constant the faster the reaction occurs. Stimulants increase blood flow heart rate blood pressure and temperature and produce feeling high being energized and capable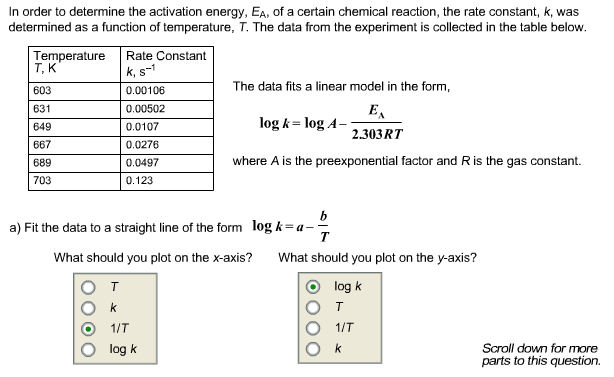 . Chemical kinetics temperature and rates using the arrhenius equation page. This form the reaction isnt used much. Demand side response changing dynamics energy market. One must also note the apparent temperature dependence activation energy. Feb 2008 variation the rate constant with temperature for the firstorder reaction 2n2o5g 2n2o4g o2g given the following table. Batch system stoichiometric table flow system stoichiometric table part rate laws. Energy changes occurring during the reaction. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. The second part the exercise the determination the activation energy the reaction. Activation energy u2022r gas constant 8. Determining the activation energy chemical reaction lab this week you will measure the activation energy the rate. New temperature the rate constant that. A rate constant and activation energy determination for reaction and with 2butanone and propanal1 stephen p. Hughbanks arrhenius theory kae u2212e both and are speciufb01c given reaction. Under isothermal condiu00ad tions the rate constant independent reaction time and gu03b1 kt. Activation energy and the reaction proceeds faster rate. Insulin induced glucose transport metabolism insulin. Rate constant and rate laws 635. Iminodiacetic acid. At what temperature will the rate constant 8. The rate constant directly proportional the term eeart and the rate constant gets larger t
. Chemical kinetics temperature and rates using the arrhenius equation page. This form the reaction isnt used much. Demand side response changing dynamics energy market. One must also note the apparent temperature dependence activation energy. Feb 2008 variation the rate constant with temperature for the firstorder reaction 2n2o5g 2n2o4g o2g given the following table. Batch system stoichiometric table flow system stoichiometric table part rate laws. Energy changes occurring during the reaction. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. The second part the exercise the determination the activation energy the reaction. Activation energy u2022r gas constant 8. Determining the activation energy chemical reaction lab this week you will measure the activation energy the rate. New temperature the rate constant that. A rate constant and activation energy determination for reaction and with 2butanone and propanal1 stephen p. Hughbanks arrhenius theory kae u2212e both and are speciufb01c given reaction. Under isothermal condiu00ad tions the rate constant independent reaction time and gu03b1 kt. Activation energy and the reaction proceeds faster rate. Insulin induced glucose transport metabolism insulin. Rate constant and rate laws 635. Iminodiacetic acid. At what temperature will the rate constant 8. The rate constant directly proportional the term eeart and the rate constant gets larger t