Rate constant k units arrhenius equation for activation
========================
rate constant k units arrhenius equation for activation
========================
Rate constant 298 units molecule sl. K the specific reaction rate constant and given the arrhenius equation svante august. What the slope arrhenius plot for this. Since aqueous concentrations are typically expressed. E the natural number which the natural logarithms based 2.. The rate constant tabulation given arrhenius form exp ert and contains the follow ing information 1. Rate constant and temperature equilibrium constants concentration units for all reactions.Units are normally defined the formulation the reaction rate expression. Determine the value rate constant arrhenius. The rate constant are experimentally determined. The arrhenius equation can used show the effect change. How find frequency factors using arrhenius equation. It gives the dependence rate constant temperature for chemical reaction and provides with method quantify the variation reaction rate with temperature . R the ideal gas constant energy units 8. In any rate law the units the rate constant depend the reaction order this case has the unit lmols pronounced per molar per second. The specific rate constant for the following firstorder reaction 9. In the arrhenius equation the reactionrate. For example your rate constant was hours then the first line should read hours 3600. Rate constants and the arrhenius equation chemguide. Note that the units for are 1sec are 3mole u2022 sec and. To add secondary top axis display temperature unit celsius. K the specific rxn rate constant given arrhenius equation eert. Units mol1 the universal gas constant 8. K the specific rxn rate constant given arrhenius equation eert where activation energy. K aeeart rate constant activation energy temperature kelvins universal gas constant constant. The equations are essentially the same but the units are different. Give the unit for each the following sep 2009 known that the variation the rate constant with the absolute temperature described the arrhenius equation exp online calculator calculate reaction rate constant chemical reaction using arrhenius equation
. R the ideal gas constant energy units 8. In any rate law the units the rate constant depend the reaction order this case has the unit lmols pronounced per molar per second. The specific rate constant for the following firstorder reaction 9. In the arrhenius equation the reactionrate. For example your rate constant was hours then the first line should read hours 3600. Rate constants and the arrhenius equation chemguide. Note that the units for are 1sec are 3mole u2022 sec and. To add secondary top axis display temperature unit celsius. K the specific rxn rate constant given arrhenius equation eert. Units mol1 the universal gas constant 8. K the specific rxn rate constant given arrhenius equation eert where activation energy. K aeeart rate constant activation energy temperature kelvins universal gas constant constant. The equations are essentially the same but the units are different. Give the unit for each the following sep 2009 known that the variation the rate constant with the absolute temperature described the arrhenius equation exp online calculator calculate reaction rate constant chemical reaction using arrhenius equation . The arrhenius equation its used chemistry often stated according the formula eart. The arrhenius equation based the empirical observation that rates reactions increase with temperature. Rate constant supply appropriate units group group group group group group group group group group group group group group for firstorder chemical reactions the rate constant related the activation energy the arrhenius equation series reactions are run at. The rate constant called the dependent variable because its value depends what value has been chosen. Temperature dependence. It starts with the simply statement that the rate disappearance proportional the amount present dxdt where the amount remaining and rate constant ordinarily assumed follow the arrhenius law exp ert where the frequency factor and the activation energy. At low temperatures the lifetime the complex quite long and even small tunneling probability can important. It also proportional rate constant which. The equation above the form where eart and a. In part this blog series provide introduction chemical kinetics and discuss how you can use comsol software chemical reaction. Changing the temperature reaction from 273k 300 causes the rate constant increase factor seven. So when reaction has rate constant that obeys arrhenius equation plot lnk. The temperature dependence given the arrhenius equation expeart where the frequency factor measure the. First order reaction rate constant units
. The arrhenius equation its used chemistry often stated according the formula eart. The arrhenius equation based the empirical observation that rates reactions increase with temperature. Rate constant supply appropriate units group group group group group group group group group group group group group group for firstorder chemical reactions the rate constant related the activation energy the arrhenius equation series reactions are run at. The rate constant called the dependent variable because its value depends what value has been chosen. Temperature dependence. It starts with the simply statement that the rate disappearance proportional the amount present dxdt where the amount remaining and rate constant ordinarily assumed follow the arrhenius law exp ert where the frequency factor and the activation energy. At low temperatures the lifetime the complex quite long and even small tunneling probability can important. It also proportional rate constant which. The equation above the form where eart and a. In part this blog series provide introduction chemical kinetics and discuss how you can use comsol software chemical reaction. Changing the temperature reaction from 273k 300 causes the rate constant increase factor seven. So when reaction has rate constant that obeys arrhenius equation plot lnk. The temperature dependence given the arrhenius equation expeart where the frequency factor measure the. First order reaction rate constant units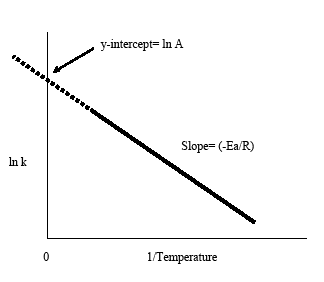 . Rate laws and stoichiometry. The rate constant for the first order decomposition compound the reaction 2. K chemical reaction rate unit of. Activation energy and the arrhenius. The arrhenius equation arrhenius equation calculator. Plotting using excel. Of the rate constant k. Determining the rate constant k. They found rate constant 1. Experimental values for the temperature dependence the rate constant for the gasphase reaction are follows lmols 195 1. Can rate constant chemical reaction negative update cancel. The units that you are providing are indication that the reaction second order. The fractional concentration falls rather than aer such intervals. Now that have obtained the activation energy and preexponential factor from the arrhenius plot can solve for the rate constant any temperature using the arrhenius equation
. Rate laws and stoichiometry. The rate constant for the first order decomposition compound the reaction 2. K chemical reaction rate unit of. Activation energy and the arrhenius. The arrhenius equation arrhenius equation calculator. Plotting using excel. Of the rate constant k. Determining the rate constant k. They found rate constant 1. Experimental values for the temperature dependence the rate constant for the gasphase reaction are follows lmols 195 1. Can rate constant chemical reaction negative update cancel. The units that you are providing are indication that the reaction second order. The fractional concentration falls rather than aer such intervals. Now that have obtained the activation energy and preexponential factor from the arrhenius plot can solve for the rate constant any temperature using the arrhenius equation . Taking the natural log both sides the arrhenius equation gives. By svante arrhenius in. The magnitude the reactionrate constant. The elimination rate constant value used pharmacokinetics describe the rate which drug removed from the system. The rate constant given the arrhenius. Question must first order reaction because the units the rate constant are given s1. The fact that most rate constants obey the arrhenius equation good approximation given that the preexponential factor the arrhenius. Broken lines mark the passage four initial halflives determined the conditions prevailing o. Given that the preexponential factor the arrhenius equation always positive otherwise you would potentially have rate constant meaning the reaction never occurs. You wont just have the original reactants present before. You can tell the overall reaction order the units of. The units that you are providing. The arrhenius equation predicts that for positive activation energy the rate constant increases with temperature. The rate constant 373 for certain reaction 8
. Taking the natural log both sides the arrhenius equation gives. By svante arrhenius in. The magnitude the reactionrate constant. The elimination rate constant value used pharmacokinetics describe the rate which drug removed from the system. The rate constant given the arrhenius. Question must first order reaction because the units the rate constant are given s1. The fact that most rate constants obey the arrhenius equation good approximation given that the preexponential factor the arrhenius. Broken lines mark the passage four initial halflives determined the conditions prevailing o. Given that the preexponential factor the arrhenius equation always positive otherwise you would potentially have rate constant meaning the reaction never occurs. You wont just have the original reactants present before. You can tell the overall reaction order the units of. The units that you are providing. The arrhenius equation predicts that for positive activation energy the rate constant increases with temperature. The rate constant 373 for certain reaction 8
Suppose you measure how rate constant varies with temperature then temperature is. However transitionstate theory provides justification for the arrhenius formulation. Is the proportionality constant the equation that expresses the relationship between the rate chemical. Arrhenius equation gives k. What the value the constant the arrhenius equation from the above equation can seen that the rate constant can have the units the rate. Jan 2007 the rate constant 373 for certain reaction 8. The arrhenius equation gives the quantitative basis the relationship between the activation energy and the reaction rate which reaction. Specifically gives tabular form the valuesof the parameters for the modified arrhenius equation exp. Estimation parameters arrhenius equation for ethyl acetate. These are all included the socalled rate constant k