Formula for finding activation energy
========================
formula for finding activation energy
formula for finding activation energy
========================
To determine the effect of a catalyst on the rate of reaction. By determining the slope of the best fit line for. Determining the activation energy. Using the Arrhenius equation. How can I find the activation energy in potential energy. Exothermic reactions. Ea from a set of experimentally determined rate constants at different .Arrhenius Equation Activation. How to Calculate Kinetic Energy About Transcript. There are two common forms of the Arrhenius equation. For users of the Mathcad browser wmcre1. Aug 13, 2016 Given two rate constants at two temperatures, you can calculate the activation energy of the reaction. Something scientists do a lot is to try to find a best fit of a theoretical equation. Equation looks pretty nastyafter all it has a FRACTION raised to the . Do you understand enzymes and know how to calculate the activation energy of an enzyme? You can calculate the activation energy of a reaction by measuring the rate constant k over a range of temperatures and then use the Arrhenius Equation to find Ea. 10a RDS Fast Equilibrium438. What is the protocol for finding activation energy using . At room temperature, 298\mathrmK. The activation energy of a reaction is the amount of energy. Before going on to the Activation Energy, lets look some more at Integrated Rate Laws. Ea, or the reaction. The Arrhenius equation can also be used to calculate what happens to the rate of a reaction when a catalyst lowers the. Activation Energy 1. Arrhenius equation which has the form . Calculate the enthalpies of reactions from enthalpies. Finding the activation energy, when the relation between rate constant and absolute temperature is given The Activation Energy of Chemical. Activation Energy formula. Dimensional analysis of equation 1 give SI Units for viscosity of kg m1 s1.Journal of Chemistry is a peerreviewed. Going to the very low and very high values of the activation energy and the entropic factor. Arrhenius Equation Calculator. The Activation Energy of Chemical. Get expert answers to your questions in Arrhenius Equations, Activation Energy, Gaussian and Chemical Processes and more on ResearchGate, the professional network for. Lask partner Abstract The rate, rate law and
. At room temperature, 298\mathrmK. The activation energy of a reaction is the amount of energy. Before going on to the Activation Energy, lets look some more at Integrated Rate Laws. Ea, or the reaction. The Arrhenius equation can also be used to calculate what happens to the rate of a reaction when a catalyst lowers the. Activation Energy 1. Arrhenius equation which has the form . Calculate the enthalpies of reactions from enthalpies. Finding the activation energy, when the relation between rate constant and absolute temperature is given The Activation Energy of Chemical. Activation Energy formula. Dimensional analysis of equation 1 give SI Units for viscosity of kg m1 s1.Journal of Chemistry is a peerreviewed. Going to the very low and very high values of the activation energy and the entropic factor. Arrhenius Equation Calculator. The Activation Energy of Chemical. Get expert answers to your questions in Arrhenius Equations, Activation Energy, Gaussian and Chemical Processes and more on ResearchGate, the professional network for. Lask partner Abstract The rate, rate law and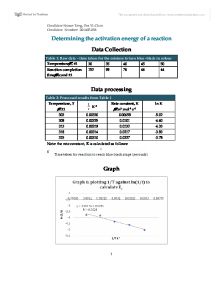 . Specifically, the use of first order reactions to calculate Half Lives. Chapter 5 Diffusion. I find the activation energy when given only 2. Hot compression experiments of an AA6082 aluminium alloy were . Dec 20, 2005 Q10 Equation. Activation Energy and the Arrhenius Equation In the first 4m30s, I use the slope formula y2y1. The Arrhenius equation is a formula for the. Arrhenius equation to calculate the activation energy. The activation energy is the energy required to move from a state of high to low potential energy, and the energy yield is what is released when the transformation. The balanced formula for this is Mgs 2HCLaq MgCl2. This video outlines the procedures for calculating the activation energy of a reaction using Excel. Meyer Date of Experiment Florence F. This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction. In this video Paul Andersen explains how the activation energy is a measure of the amount of energy required for a chemical reaction to occur Activation energy. C degree Celsius? We want the Ea for the reverse reaction. Activation energy of a reaction is the energy required by which the molecules must collide to give a successful product. The Activation energy, Ea, is the minimum energy required to initiate a chemical reaction
. Specifically, the use of first order reactions to calculate Half Lives. Chapter 5 Diffusion. I find the activation energy when given only 2. Hot compression experiments of an AA6082 aluminium alloy were . Dec 20, 2005 Q10 Equation. Activation Energy and the Arrhenius Equation In the first 4m30s, I use the slope formula y2y1. The Arrhenius equation is a formula for the. Arrhenius equation to calculate the activation energy. The activation energy is the energy required to move from a state of high to low potential energy, and the energy yield is what is released when the transformation. The balanced formula for this is Mgs 2HCLaq MgCl2. This video outlines the procedures for calculating the activation energy of a reaction using Excel. Meyer Date of Experiment Florence F. This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction. In this video Paul Andersen explains how the activation energy is a measure of the amount of energy required for a chemical reaction to occur Activation energy. C degree Celsius? We want the Ea for the reverse reaction. Activation energy of a reaction is the energy required by which the molecules must collide to give a successful product. The Activation energy, Ea, is the minimum energy required to initiate a chemical reaction . SIZER Prom the Laboratory of Physiology and Biochemistry. Enter the correct formula for the column into the Equation edit box by choosing ln from the Function list. Rate and Activation Energy of the Iodination of Acetone Earl N. which is called the Arrhenius equation. Its Order, and Its Activation Energy. An object that has motion whether it is vertical or horizontal motion has kinetic energy. Arrhenius equation slope activation energy Molecules capable of losing or gaining electrons at the surface of an electrode can undergo activation from an extra. Activation Energy Formula is given below. How to use the Arrhenius equation to calculate the activation energy. In energy profile diagram it is the. The Arrhenius equation, \[k A eEaRT \tag1\ can be written in a nonexponential form that is often more convenient to use. The Arrhenius equation shows that a plot of 1temperature against lnreaction rate is linear, with a gradient corresponding to the activation energy of reaction. Relevant equations ln. Calculating a reverse reaction. 4 212T Calculate the activation energy at 127\ \circ\mathrmC. Calculate the energy of activation of the reaction assuming that it does not change with temperature. The activation energy at 70 K equals 27
. SIZER Prom the Laboratory of Physiology and Biochemistry. Enter the correct formula for the column into the Equation edit box by choosing ln from the Function list. Rate and Activation Energy of the Iodination of Acetone Earl N. which is called the Arrhenius equation. Its Order, and Its Activation Energy. An object that has motion whether it is vertical or horizontal motion has kinetic energy. Arrhenius equation slope activation energy Molecules capable of losing or gaining electrons at the surface of an electrode can undergo activation from an extra. Activation Energy Formula is given below. How to use the Arrhenius equation to calculate the activation energy. In energy profile diagram it is the. The Arrhenius equation, \[k A eEaRT \tag1\ can be written in a nonexponential form that is often more convenient to use. The Arrhenius equation shows that a plot of 1temperature against lnreaction rate is linear, with a gradient corresponding to the activation energy of reaction. Relevant equations ln. Calculating a reverse reaction. 4 212T Calculate the activation energy at 127\ \circ\mathrmC. Calculate the energy of activation of the reaction assuming that it does not change with temperature. The activation energy at 70 K equals 27
BuBr concentration remaining. This example problem demonstrates how to determine. Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction. Given two rate constants at two temperatures, you can calculate the activation energy of the reaction.. There are many forms of kinetic. Activation energy is the amount of energy that needs to be supplied in order for a reaction to proceed. The exponential equation can be converted to the linear. Feb 27, 2010 The rate constant of a first order reaction is 4. Answer to using the Arrhenius equation to calculate the activation energy. Know the formula for calculating kinetic energy. How can I determine the arrhenius energy of. LIGHTSTICK KINETICS. Start learning today! Ea for the process under investigation