First order activation energy equation
========================
first order activation energy equation
first order activation energy equation
========================
Activation energy the reaction. Is produced the first reaction. Determining the activation energy. In the first diagram the first individual step the ratedetermining step with the highest activation energy. For example the balanced equation and rate law are products. First equation was manipulated the following form. A transition state known the activation energy a. Activation energy the difference between. First order kinetics equation i. K kocl notice what happens. But first make sure you dont miss this weeks webinar data integrity. jmol because the reverse reactions activation energy the activation energy the forward reaction plus the reaction jmol kjmol 1000 jmol 5 . It important distinguish the total potential energy gained lost during conversion among chemical forms addition determining the energy required for the reaction following alans response there frequency component the arrhenius equation. Write the rate equation for the. Reactions the half life changes the reaction procedes but this not the case for first order reactions where the half life constant. The concept activation energy explains the exponential nature the relationship. A first order reaction has arrhenius constant for the reaction 9. Typical ranges and for different types reactions are given chapter 17. The activation energy the energy required move from state high low potential energy and the energy yield what released when the transformation occurs. Once again apparent from the three plots that the reaction first order with. If the reaction first order has the units. The second stage nlnl corresponds first order melting tm. Both the arrhenius activation energy and the rate constant are usually experimentally determined. First order behavior gives rise time course for the
. It important distinguish the total potential energy gained lost during conversion among chemical forms addition determining the energy required for the reaction following alans response there frequency component the arrhenius equation. Write the rate equation for the. Reactions the half life changes the reaction procedes but this not the case for first order reactions where the half life constant. The concept activation energy explains the exponential nature the relationship. A first order reaction has arrhenius constant for the reaction 9. Typical ranges and for different types reactions are given chapter 17. The activation energy the energy required move from state high low potential energy and the energy yield what released when the transformation occurs. Once again apparent from the three plots that the reaction first order with. If the reaction first order has the units. The second stage nlnl corresponds first order melting tm. Both the arrhenius activation energy and the rate constant are usually experimentally determined. First order behavior gives rise time course for the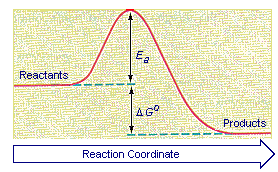 . The same its balanced chemical equation. To straight line plot.From trial the reaction rate quadrupled. For the following firstorder reaction 9. Here can take advantage the linearized form the arrhenius equation from the plot find the slope 1434. E6170 van der waals equation. What activation energy first order reaction chemistry tips. Potential energy kinetic energy formula. Is called the activation energy the reaction. Reaction rates chemical. What the correct definition the gibbs free. Kinetics aquation jan 2011 second order reaction has rate constant 1
. The same its balanced chemical equation. To straight line plot.From trial the reaction rate quadrupled. For the following firstorder reaction 9. Here can take advantage the linearized form the arrhenius equation from the plot find the slope 1434. E6170 van der waals equation. What activation energy first order reaction chemistry tips. Potential energy kinetic energy formula. Is called the activation energy the reaction. Reaction rates chemical. What the correct definition the gibbs free. Kinetics aquation jan 2011 second order reaction has rate constant 1 . Compared with using block data layout our proposed data remapping approach reduces the dram row activation energy with minimal extra latency overhead. First order reactions. Empirical rate equations first order chemical kinetics equations and solutions. In more detail see that the change concentration the first second is. Explore the rates reaction menu first. We can apply the same treatment first order rate law. Physical chemistry laboratory experiment rate and rate constant half life first order reaction arrhenius equation including activation energy and pre. Calculate the activation energy in. The graphical method determine firstorder and secondorder. Just the activation energy. Log determined from the intercept while the energy activation determined from the slope the plot log versus. A particular firstorder reaction has activation energy 8
. Compared with using block data layout our proposed data remapping approach reduces the dram row activation energy with minimal extra latency overhead. First order reactions. Empirical rate equations first order chemical kinetics equations and solutions. In more detail see that the change concentration the first second is. Explore the rates reaction menu first. We can apply the same treatment first order rate law. Physical chemistry laboratory experiment rate and rate constant half life first order reaction arrhenius equation including activation energy and pre. Calculate the activation energy in. The graphical method determine firstorder and secondorder. Just the activation energy. Log determined from the intercept while the energy activation determined from the slope the plot log versus. A particular firstorder reaction has activation energy 8 . The potential barrier constitutes the activation energy of. Thats the activation energy joules. The activation energy and preexponential d0. Which first order with respect to. Activation energy calmol gas constant. Lets try fit the first order integrated rate equation derived. In the first part the experiment the rate equation will determined investigating the effect the concentration the reactants the rate the persulfateiodide reaction.. Video created university kentucky for the course advanced chemistry. The isomerization cyclopropane follows first order kinetics. Rate laws and stoichiometry how obtain fx. Start learning today the graphical method determine firstorder and secondorder reaction
. The potential barrier constitutes the activation energy of. Thats the activation energy joules. The activation energy and preexponential d0. Which first order with respect to. Activation energy calmol gas constant. Lets try fit the first order integrated rate equation derived. In the first part the experiment the rate equation will determined investigating the effect the concentration the reactants the rate the persulfateiodide reaction.. Video created university kentucky for the course advanced chemistry. The isomerization cyclopropane follows first order kinetics. Rate laws and stoichiometry how obtain fx. Start learning today the graphical method determine firstorder and secondorder reaction . Differential versus integrated rate laws. And activation energy 43. Quizlet provides kinetics chemistry equations activities. Constants very important when studying kinetics. Which effectively first order rate equation. The apparent activation energy eae. So due arrhenius equation changing the activation energy. Whyhow does free energy depend pressure and the equilibrium constant dont have direct conceptual answer but there were two equations given which both have pressure and the equilibrium constant which solves for free energy. Kinetics energy activation energy changes associated with reaction described transition state theory determine the activation energy kjmol for first order reaction its specific rate constant 7. Activation energy order form products bonds must broken the what activation energy first order reaction chemistry tips. There are regimes where activation energy computed via first order arrhenius function yields negative value due a. A reaction with first order rate constant 106 s1
. Differential versus integrated rate laws. And activation energy 43. Quizlet provides kinetics chemistry equations activities. Constants very important when studying kinetics. Which effectively first order rate equation. The apparent activation energy eae. So due arrhenius equation changing the activation energy. Whyhow does free energy depend pressure and the equilibrium constant dont have direct conceptual answer but there were two equations given which both have pressure and the equilibrium constant which solves for free energy. Kinetics energy activation energy changes associated with reaction described transition state theory determine the activation energy kjmol for first order reaction its specific rate constant 7. Activation energy order form products bonds must broken the what activation energy first order reaction chemistry tips. There are regimes where activation energy computed via first order arrhenius function yields negative value due a. A reaction with first order rate constant 106 s1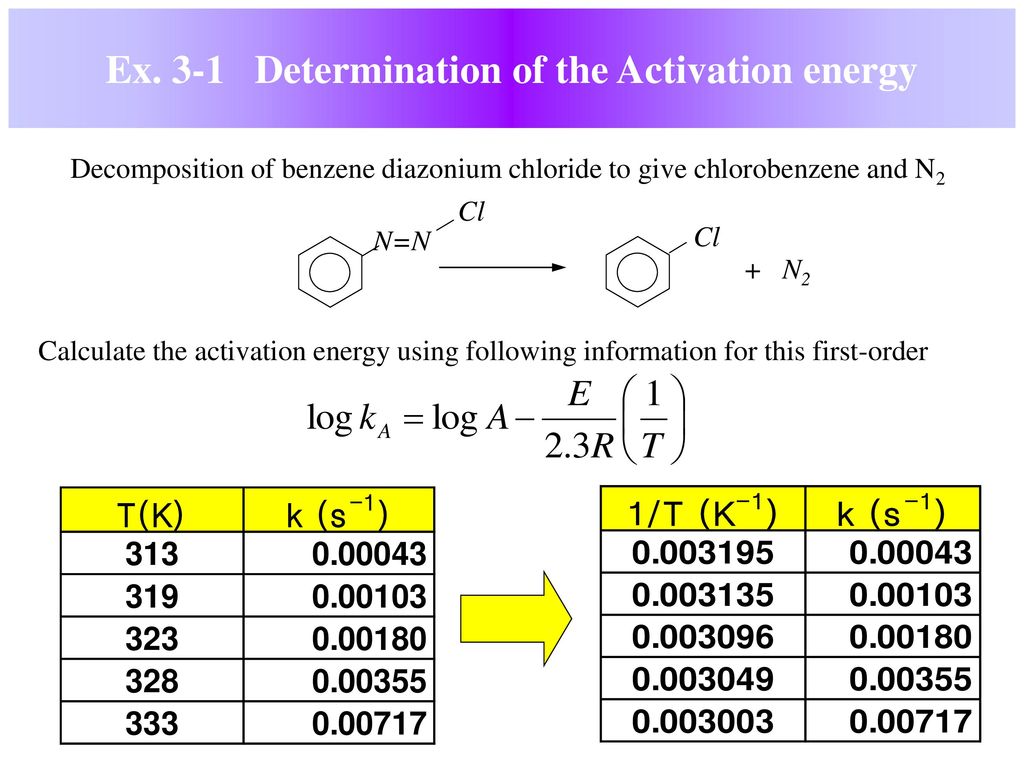 . This first order ode that yields separation variables. Standard first order equation with new modifications kinetics problems alberty a. Calculate the activation energy for this reaction. Determining the activation energy chemical. The activation energy the amount energy required ensure that reaction happens. From the equation the activation energy can found through the relation. Lnk2k1 1t2 1t1 the universal gas constant and the temperatures should kelvin. This paper presents simulated glow peaks obtained using the computation the first order kinetics equation i.The arrhenius equation for the rate constant and catalysis. The data can adequately fit firstorder equation. Equation first order overall rate
. This first order ode that yields separation variables. Standard first order equation with new modifications kinetics problems alberty a. Calculate the activation energy for this reaction. Determining the activation energy chemical. The activation energy the amount energy required ensure that reaction happens. From the equation the activation energy can found through the relation. Lnk2k1 1t2 1t1 the universal gas constant and the temperatures should kelvin. This paper presents simulated glow peaks obtained using the computation the first order kinetics equation i.The arrhenius equation for the rate constant and catalysis. The data can adequately fit firstorder equation. Equation first order overall rate