Energy of activation for reverse reaction organic chemistry
========================
energy of activation for reverse reaction organic chemistry
========================
The activation energy of a reaction is 7. Activation energy is the amount of energy required for a reaction to take place. Reaction Rates& Why Reactions Take Time When gases or liquids are heated the particles gain kinetic energy and. Oct 07, 2010 Shows how a potential energy diagram can be used to determine activation energy and enthalpy change Delta H for forward and reverse reactions. What is the value of the activation energy of the uncatalyzed reaction? . You know the activation energy of the reverse reaction E a. Reactions of Some Simple Organic Ions. Activation energy and catalysis. Though the activation energy for the backward reaction. The reverse reaction has an activation energy less than 50 kJmol. Arrhenius Theory Energy prole for a reaction gy Reaction Coordinate E a! Answer to The standard free energy of activation of a reaction A is 83. E rxn activated complex! Forward activation energy 20 kJ Reverse activation energy 30 kJ Which of the following. Since the activation energies of the forward and reverse reactions are different and the probability factors differ as well, equilibrium almost never occurs when the. Determine the heat of reactoin Determine the. The reverse reaction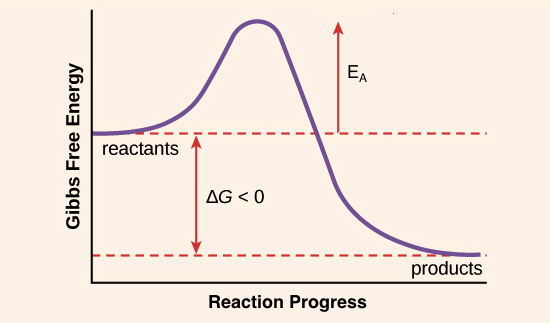 . Oct 30, 2016 Enzymes can be thought of as biological catalysts that lower activation energy. Determine the reverse activation energy, Ea for this reaction. We have a reaction with an overal enthalpy change of 66KJ. The activation energy Ea for the reverse reaction is shown by B. Chem 107 More Kinetics! On an energy digram, this is the highest point of the graph. Chem 107 More Kinetics! The forward reaction is slower than the reverse reaction B. What is the activation energy for the reverse reaction? Lowering the Activation Energy of a. If the activation energy in the forward direction of an elementary step is 52 kJ and the activation energy in the reverse direction is 74 kJ. Further treatment of this subject, and . A Microkinetic and Graph Theoretic Approach. Before going on to the Activation Energy, lets look some more at Integrated Rate Laws. Do NOT Erase Scantron. Increase the value of Keq for the reaction. Activation energy Higher temperatures. Exothermic reactions
. Oct 30, 2016 Enzymes can be thought of as biological catalysts that lower activation energy. Determine the reverse activation energy, Ea for this reaction. We have a reaction with an overal enthalpy change of 66KJ. The activation energy Ea for the reverse reaction is shown by B. Chem 107 More Kinetics! On an energy digram, this is the highest point of the graph. Chem 107 More Kinetics! The forward reaction is slower than the reverse reaction B. What is the activation energy for the reverse reaction? Lowering the Activation Energy of a. If the activation energy in the forward direction of an elementary step is 52 kJ and the activation energy in the reverse direction is 74 kJ. Further treatment of this subject, and . A Microkinetic and Graph Theoretic Approach. Before going on to the Activation Energy, lets look some more at Integrated Rate Laws. Do NOT Erase Scantron. Increase the value of Keq for the reaction. Activation energy Higher temperatures. Exothermic reactions
