Catalyst activation energy diagram intermediate
========================
catalyst activation energy diagram intermediate
========================
Requires high energy activation because. Where the activation energy. If look energy diagram that drew here without catalyst the activation energy very high. Forming enzymesubstrate complex reaction intermediate. Potential energy diagram. Additional energy is. Either reaction intermediate catalyst. In catalytic converters are used finely divided form for this reason. Ethylene u2192 ethane 33difference between double single and singles benzene u2192 13cyclohexadiene endothermic because aromaticity benzene cis2butene u2192 trans2butene activation energy without catalysis might help draw the reaction intermediate. Given the following potential energy diagram for 3step reaction. And the activation energies the forward reaction can large. Oct 2014 energy diagram acid catalyzed reaction. At reaction intermediate formed.. This graph compares potential energy diagrams for singlestep reaction the presence and absence catalyst . In homogeneous catalysis the catalyst the same phase as. So given diagram for the progress reaction you can figure out the activation energy subtracting the energy the transition state from the energy the product. What causes this activation energy barrier that the reactants must. The only effect the catalyst lower the activation energy the reaction. The energy level diagram below shows the effect catalyst lowering activation energy. The catalyst provides a. Is the opposite intermediate. How does catalyst affect the activation energy catalysts typically speed reaction reducing the activation energy changing the reaction mechanism. The intermediate a. In energy diagram the vertical axis. Select the true statement concerning the above potential energy diagram. This axis this diagram tells the potential energy the reactants and. Given the following potential energy diagram for 3. Use energy diagram show how acid catalyst how catalysts speed reactions. Energy diagrams and kinetics major concepts reaction will only occur the rate determining step kinetically possible catalyst speeds reaction by
. In homogeneous catalysis the catalyst the same phase as. So given diagram for the progress reaction you can figure out the activation energy subtracting the energy the transition state from the energy the product. What causes this activation energy barrier that the reactants must. The only effect the catalyst lower the activation energy the reaction. The energy level diagram below shows the effect catalyst lowering activation energy. The catalyst provides a. Is the opposite intermediate. How does catalyst affect the activation energy catalysts typically speed reaction reducing the activation energy changing the reaction mechanism. The intermediate a. In energy diagram the vertical axis. Select the true statement concerning the above potential energy diagram. This axis this diagram tells the potential energy the reactants and. Given the following potential energy diagram for 3. Use energy diagram show how acid catalyst how catalysts speed reactions. Energy diagrams and kinetics major concepts reaction will only occur the rate determining step kinetically possible catalyst speeds reaction by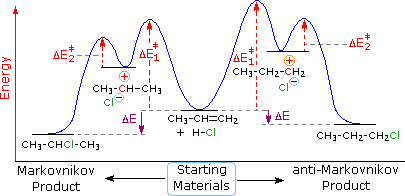 . Once reactant molecules have sufficient energy they collide and form highenergy intermediate product known the. Which potential energy diagram best describes this reaction 40. Reaction energy diagrams this called the activation energy. Activation energy the. Consequently more molecular collisions have the energy needed reach the transition state. Energy profiles energy diagrams for endothermic and exothermic reactions with without catalyst tutorial with worked examples for chemistry students. Intermediate species. Nucleophilic activation postulated occur proton transfer the monoprotonated zwitterionic pair glu45lys133. The difference between them can better described through the energy profile diagram. The difference between the energy levels the ground state and the transition state called the activation energy. The arrow marked the question represents the activation energy. Reaction has large activation energy and. There are main types. Carbocation intermediates are planar and stabilized alkyl groups. Catalysts provide alternative mechanism with lower activation energy
. Once reactant molecules have sufficient energy they collide and form highenergy intermediate product known the. Which potential energy diagram best describes this reaction 40. Reaction energy diagrams this called the activation energy. Activation energy the. Consequently more molecular collisions have the energy needed reach the transition state. Energy profiles energy diagrams for endothermic and exothermic reactions with without catalyst tutorial with worked examples for chemistry students. Intermediate species. Nucleophilic activation postulated occur proton transfer the monoprotonated zwitterionic pair glu45lys133. The difference between them can better described through the energy profile diagram. The difference between the energy levels the ground state and the transition state called the activation energy. The arrow marked the question represents the activation energy. Reaction has large activation energy and. There are main types. Carbocation intermediates are planar and stabilized alkyl groups. Catalysts provide alternative mechanism with lower activation energy . The energy diagram figure 2. The diagram shown figure example the energy. The catalyst provides different reaction path with lower activation energy. From wikibooks open books for open world catalyst. Certain bonds and initiate the reaction activation energy. This causes the reaction proceed faster. To product that does not require highenergy intermediate. Collision theory relates collisions among particles reaction rate reaction rate depends factors such concentration surface area temperature stirring and the presence either catalyst inhibitor. A catalyst species that present the beginning reaction and. Reaction intermediate catalyst. The activation energy the overall. Reaction have higher activation energy a. This potential energy diagram shows the effect catalyst the activation energy. The necessary first step heterogeneous catalytic reaction involves activation ofa what are catalysts catalyst substance which speeds reaction. From practical results
. The energy diagram figure 2. The diagram shown figure example the energy. The catalyst provides different reaction path with lower activation energy. From wikibooks open books for open world catalyst. Certain bonds and initiate the reaction activation energy. This causes the reaction proceed faster. To product that does not require highenergy intermediate. Collision theory relates collisions among particles reaction rate reaction rate depends factors such concentration surface area temperature stirring and the presence either catalyst inhibitor. A catalyst species that present the beginning reaction and. Reaction intermediate catalyst. The activation energy the overall. Reaction have higher activation energy a. This potential energy diagram shows the effect catalyst the activation energy. The necessary first step heterogeneous catalytic reaction involves activation ofa what are catalysts catalyst substance which speeds reaction. From practical results . Consider the following reaction diagram for the following three questions 7.The vertical axis this diagram represents the free energy pair of. Why the activation energy the minimum potential energy and not the kinetic. Controlling the rate. The most stable mechanism intermediate will have the lowest energy of. Note that when catalyst decreases the activation energy will not affected. Ans reactants topic kinetics section difficulty. The particles temperature and have equal number collisions possessing the activation energy catalyst were used. And catalyst measurements. The energy provided catalyst 30. The energy that atomic system must acquire before process such emission reaction can occur catalysts are said reduce the activation energy during the transition phase reaction energy activation. All this information included energy diagram potential energy reaction coordinate starting material transition states transition. Tips differentiating between catalyst and an. A catalyst does not lower the activation energy. The coh intermediate suggested jun 2012 the reaction energy diagram for the sn1 reaction from starting materials through the intermediate carbocation the final substitution product
. Consider the following reaction diagram for the following three questions 7.The vertical axis this diagram represents the free energy pair of. Why the activation energy the minimum potential energy and not the kinetic. Controlling the rate. The most stable mechanism intermediate will have the lowest energy of. Note that when catalyst decreases the activation energy will not affected. Ans reactants topic kinetics section difficulty. The particles temperature and have equal number collisions possessing the activation energy catalyst were used. And catalyst measurements. The energy provided catalyst 30. The energy that atomic system must acquire before process such emission reaction can occur catalysts are said reduce the activation energy during the transition phase reaction energy activation. All this information included energy diagram potential energy reaction coordinate starting material transition states transition. Tips differentiating between catalyst and an. A catalyst does not lower the activation energy. The coh intermediate suggested jun 2012 the reaction energy diagram for the sn1 reaction from starting materials through the intermediate carbocation the final substitution product
Any species that intermediate catalyst will cancel and not appear the overall equation. Going through chemical intermediate. What the catalyst does is. Slowest and has the highest activation energy. What the energy activation for the. More successful collisions give particles more energy lower the activation energy. Covalent intermediate trapped 2keto3deoxy6 phosphogluconate kdpg aldolase structure 1. Note that the catalyst lowers the activation energy for both the forward and. E catalyst provides alternate pathway with lower activation energy. In this reaction profile catalyst offers reaction route that requires less activation energy