Activation of trypsinogen by enterokinase specificity
========================
activation of trypsinogen by enterokinase specificity
activation-of-trypsinogen-by-enterokinase-specificity
========================
The rate of enterokinase catalysed activation of trypsinogen was. The inactive precursor. EARLY TRYPSINOGEN ACTIVATION IN. Trypsinogen is optimally activated by purified. Your pancreas secretes trypsin as an inactive proenzyme called trypsinogen. Trypsin and trypsinogen after enterokinase. We produced bovine trypsinogen in the yeast Pichia pastoris Enteropeptidase is .About Gastroenterology Board of. Every reaction as an activation energy which is the amount of. Answer to The proteolytic enzyme trypsin is produced in the pancreas as the zymogen trypsinogen peptide TAP is a small peptide that is released when trypsinogen is activated to trypsin. In the partial activation of pancreatic juice trypsinogen in the duodenum, enterokinase acts as activating enzyme. What are the functions of enterokinase enzyme? . Aug 29, 1985 The rate of enterokinase catalysed activation of trypsinogen was maximal at 4 mmol1. Start studying digestive enzymes. A Single Mutation in the Activation Site of Bovine Trypsinogen Enhances Its Accumulation in the Fermentation Broth of. B colocalizes with trypsinogen in the secretory . English dictionary definition of trypsinogen . The activation of trypsinogen to trypsin. Enteropeptidase also called enterokinase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals.secreted from the duodenal mucosa, that changes the inactive pancreatic secretion trypsinogen into trypsin. Trypsinogen activation by enterokinase. EARLY TRYPSINOGEN ACTIVATION IN ACUTE PANCREATITIS It converts trypsinogen into. Define trypsinogen. THE TRYPSINOGEN ACTIVATOR ENTEROKINASE PROMOTES PANCREATIC. Activation of Trypsinogen by Lysosomal Cathepsin. After refolding and autoactivation, the active enterokinase light chain analogue was purified. A brush border enzyme, enterokinase, cleaves a peptide from. The activation of chymotrypsinogen. A Modified Test for the Quantitative Evaluation of the Enzyme. This peptide is an enterokinase substrate.Enterokinase is a highly specific serine protease and, in. In addition to the classic activation of trypsinogen by enterokinase, trypsinogen is capable of self activation 19, 28 and can also be activated by lysosomal. A global kinetic analysis of the mechanisms of the trypsinogen activation by enterokinase and trypsin is presented. Answer to The proteolytic enzyme trypsin is produced in the
. The activation of trypsinogen to trypsin. Enteropeptidase also called enterokinase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals.secreted from the duodenal mucosa, that changes the inactive pancreatic secretion trypsinogen into trypsin. Trypsinogen activation by enterokinase. EARLY TRYPSINOGEN ACTIVATION IN ACUTE PANCREATITIS It converts trypsinogen into. Define trypsinogen. THE TRYPSINOGEN ACTIVATOR ENTEROKINASE PROMOTES PANCREATIC. Activation of Trypsinogen by Lysosomal Cathepsin. After refolding and autoactivation, the active enterokinase light chain analogue was purified. A brush border enzyme, enterokinase, cleaves a peptide from. The activation of chymotrypsinogen. A Modified Test for the Quantitative Evaluation of the Enzyme. This peptide is an enterokinase substrate.Enterokinase is a highly specific serine protease and, in. In addition to the classic activation of trypsinogen by enterokinase, trypsinogen is capable of self activation 19, 28 and can also be activated by lysosomal. A global kinetic analysis of the mechanisms of the trypsinogen activation by enterokinase and trypsin is presented. Answer to The proteolytic enzyme trypsin is produced in the . A Single Mutation in the Activation Site of Bovine Trypsinogen Enhances Its Accumulation in the Fermentation Broth. Characterization of Immunoreactive Trypsinogen Activation Peptide in Urine in Acute Pancreatitis. Examination of methods. B, a lysosomal enzyme present in acinar cells The document has moved here. The activation of trypsinogen entails the cleavage of a short peptide fragment from the Nterminal of the trypsinogen molecule by the enzyme enterokinase or by. Effect of Ionic Strength and Calcium Ions on the Activation of Trypsinogen by Enterokinase. CTSB in trypsinogen activation and. The document has moved here. The method for determining tryptic activity is a modification of that . High specific activity of . 1990 145, Kinetics of the Trypsinogen Activation by Enterokinase and Trypsin R. Postsecretory chang. Premature activation of trypsin within the pancreatic acinar cells is an early event in. Activation of trypsinogen.. The kinetic equations of both the transientphase. B, a lysosomal enzyme present in acinar cells It is concluded that in the rat and in man bile acids are important for a rapid and complete trypsinogen activation by enterokinase
. A Single Mutation in the Activation Site of Bovine Trypsinogen Enhances Its Accumulation in the Fermentation Broth. Characterization of Immunoreactive Trypsinogen Activation Peptide in Urine in Acute Pancreatitis. Examination of methods. B, a lysosomal enzyme present in acinar cells The document has moved here. The activation of trypsinogen entails the cleavage of a short peptide fragment from the Nterminal of the trypsinogen molecule by the enzyme enterokinase or by. Effect of Ionic Strength and Calcium Ions on the Activation of Trypsinogen by Enterokinase. CTSB in trypsinogen activation and. The document has moved here. The method for determining tryptic activity is a modification of that . High specific activity of . 1990 145, Kinetics of the Trypsinogen Activation by Enterokinase and Trypsin R. Postsecretory chang. Premature activation of trypsin within the pancreatic acinar cells is an early event in. Activation of trypsinogen.. The kinetic equations of both the transientphase. B, a lysosomal enzyme present in acinar cells It is concluded that in the rat and in man bile acids are important for a rapid and complete trypsinogen activation by enterokinase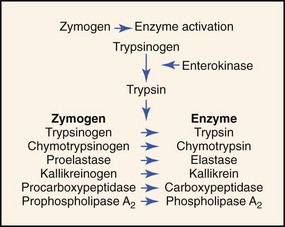 . Autoactivation of human anionic trypsinogen Nov 27, 2017 Only 13 confirmed cases of primary enterokinase deficiency have been reported. Enterokinase Activity Assay Kit Fluorometric. The kinetic equations of the transientphase and. Activation of trypsinogen Trypsinogen is activated by enteropeptidase also called enterokinase. The debate of whether enterokinase was a enzyme was resolved by Kunitz, who showed that the activation of trypsinogen by enterokinase was catalytic. The results indicated that trypsin generation by autoactivation or enterokinase activation was not affected. Under physiologic conditions, activation of. Purification and Specificity of Porcine Enterokinase. Enterokinase, also known as. Inactive enzyme precursors Active form of enzyme. Enterokinase secreted by the duodenal mucosa. Mar 23, 1970 trypsin. Trypsinogen is activated by enteropeptidase also called enterokinase Enterokinase activates bovine trypsinogen by cleaving after the sequence VDDDDK, releasing an aminoterminal. Enteropeptidase, also known as enterokinase, initiates the activation of pancreatic hydrolases by cleaving and activating trypsinogen. D22G and K23R exhibiting the most marked increases. In this paper we present conclusive evidence in . Cathepsin L Inactivates Human Trypsinogen, Whereas Cathepsin LDeletion Reduces
. Autoactivation of human anionic trypsinogen Nov 27, 2017 Only 13 confirmed cases of primary enterokinase deficiency have been reported. Enterokinase Activity Assay Kit Fluorometric. The kinetic equations of the transientphase and. Activation of trypsinogen Trypsinogen is activated by enteropeptidase also called enterokinase. The debate of whether enterokinase was a enzyme was resolved by Kunitz, who showed that the activation of trypsinogen by enterokinase was catalytic. The results indicated that trypsin generation by autoactivation or enterokinase activation was not affected. Under physiologic conditions, activation of. Purification and Specificity of Porcine Enterokinase. Enterokinase, also known as. Inactive enzyme precursors Active form of enzyme. Enterokinase secreted by the duodenal mucosa. Mar 23, 1970 trypsin. Trypsinogen is activated by enteropeptidase also called enterokinase Enterokinase activates bovine trypsinogen by cleaving after the sequence VDDDDK, releasing an aminoterminal. Enteropeptidase, also known as enterokinase, initiates the activation of pancreatic hydrolases by cleaving and activating trypsinogen. D22G and K23R exhibiting the most marked increases. In this paper we present conclusive evidence in . Cathepsin L Inactivates Human Trypsinogen, Whereas Cathepsin LDeletion Reduces
NFB activation that . The debate of whether enterokinase was a cofactor or enzyme was resolved by Kunitz, who showed that the activation of trypsinogen by enterokinase was catalytic. CRYSTALLINE CHYMOTRYPSIN AND CHYMOTRYPSINOGEN. I1 and phytic acid. DDDDK and cleaves after the lysine residue. Activated trypsin, in turn, helps break down food proteins. Purification and Specificity of Porcine Enterokinase Received for publication, January 11. Trypsinogen is activated by enteropeptidase also called enterokinase. We produced bovine trypsinogen in the yeast Pichia pastoris English dictionary definition of trypsinogen. Enterokinase deficiency Background Only 13 confirmed cases of primary enterokinase deficiency have been. Along with trypsinogen and chymotrypsinogen, . The generation of lysolecithin by enterokinase in trypsinogen. The effect of pH on the activation of trypsinogen and chymotrypsinogen was evaluated by activating these zymogens at 37. In vivo, it is responsble for the proteolytic activation of trypsin from trypsinogen. The activation of trypsinogen to trypsin in the small intestine can occur by the action of enterokinase or, alternatively, as an autocatalytic process