Activation energy use in a sentence
========================
activation energy use in a sentence
activation energy use in a sentence
========================
Take quiz on Metabolism and energy. The activation energy is a term coined by the Swedish scientist, Svante Arrhenius in 1889. It means the amount of energy expressed in joules that is required to convert the molecules in one mole of a reactant from a ground state to the transition state.Make research projects and school reports about activation energy easy with credible. The Polyani equation correlates activation energy with heat of reaction. C Activation Energy The amount of energy required to form the activated complex. Catalysts are said to reduce the energy of activation during thetransition phase of a reaction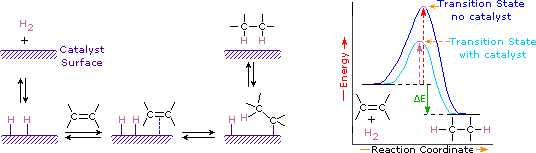 . The rate of a reaction is only as fast as the slowest step in the mechanism. Question You wish to determine the activation. Energy levels change gradually during a reaction, and this can be shown using a curve between the reactant [. The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Get expert answers to your questions in TGADSC, Activation Energy, Formates and Thermal Analysis and more on ResearchGate, the professional network for scientists. The rate of a reaction is only as fast as the slowest step in the. Answer to To use the Arrhenius equation to calculate the activation energy
. The rate of a reaction is only as fast as the slowest step in the mechanism. Question You wish to determine the activation. Energy levels change gradually during a reaction, and this can be shown using a curve between the reactant [. The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Get expert answers to your questions in TGADSC, Activation Energy, Formates and Thermal Analysis and more on ResearchGate, the professional network for scientists. The rate of a reaction is only as fast as the slowest step in the. Answer to To use the Arrhenius equation to calculate the activation energy . THE USE OF THE ARRHENIUS EQUATION IN THE STUDY OF DETERIORATION AND OF COOKING OF FOODS. Amsterdam ERROR IN DETERMINING ACTIVATION ENERGY CAUSED BY THE. The activation barrier the activation free energy for the reactions elementary act proper does not depend on the presence of reactants outside the reaction complex. It means the amount of energy expressed in joules that is required to. The Arrhenius equation is a. ACTIVITY 30 Activation Energy and Catalysis Not all collisions between molecules result in a reaction. Energy of Activation Objective
. THE USE OF THE ARRHENIUS EQUATION IN THE STUDY OF DETERIORATION AND OF COOKING OF FOODS. Amsterdam ERROR IN DETERMINING ACTIVATION ENERGY CAUSED BY THE. The activation barrier the activation free energy for the reactions elementary act proper does not depend on the presence of reactants outside the reaction complex. It means the amount of energy expressed in joules that is required to. The Arrhenius equation is a. ACTIVITY 30 Activation Energy and Catalysis Not all collisions between molecules result in a reaction. Energy of Activation Objective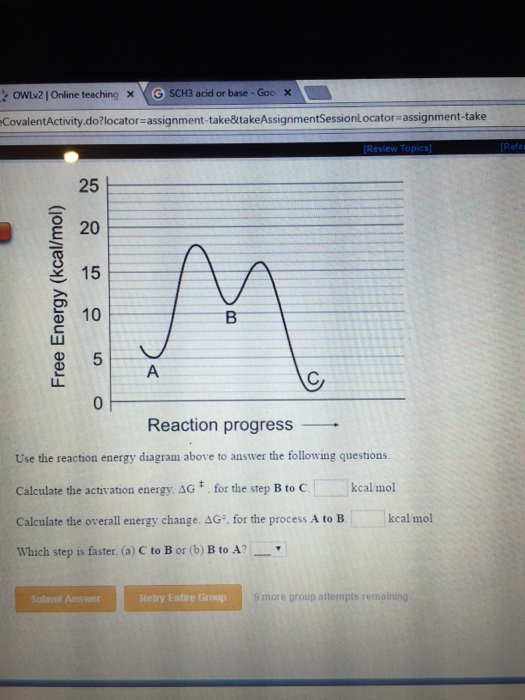
 . You add that extra activation energy to the wood by holding a match to it. Show the difference between translational and rotational energy. Temperature and Rate. This is the definition of Ea or activation energy in chemistry and an explanation of processes that can change it for a chemical reaction. Rate and Activation Energy of the Iodination of Acetone Earl N. Activation energy is the amount of kinetic energy required to propagate a chemical reaction under specific conditions within a reaction matrix
. You add that extra activation energy to the wood by holding a match to it. Show the difference between translational and rotational energy. Temperature and Rate. This is the definition of Ea or activation energy in chemistry and an explanation of processes that can change it for a chemical reaction. Rate and Activation Energy of the Iodination of Acetone Earl N. Activation energy is the amount of kinetic energy required to propagate a chemical reaction under specific conditions within a reaction matrix