Activation energy equation rate constant of a reaction
========================
activation energy equation rate constant of a reaction
activation-energy-equation-rate-constant-of-a-reaction
========================
T is the temperature. Ea is the activation energy. Definition of rate constant k, the preexponential factor A, and activation energy. I am calculating the activation energy of quartz dissolution in porcelain body. Define the term activation energy. A the preexponential frequency factor. Low Ea, High T large k faster reaction. Calculate The Activation Energy For A Reaction When Given Rate Constants Get To Know More About What Arrhenius Equation Is? How is the Activation Energy of the Arrhenius equation related to the exponents in rate laws? T is the absolute temperature. The Arrhenius equation. The reason that rates of reaction . In 1884 Vant Hoff gave the equation for . The rate constant goes on increasing as the. R is the ideal gas constant 8. Arrhenius equation and activation energy This calculator calculates the effect of temperature on reaction rates using the Arrhenius equation Ea. Of Rate Constant With Temperature. Both the Arrhenius activation energy and the rate constant k are experimentally. He showed that the expression of the rate constant given by the Arrhenius equation had. These are all included in the socalled rate constant which is only actually. E a is the activation energy! . Lets substitute some typical values into this equation
. In 1884 Vant Hoff gave the equation for . The rate constant goes on increasing as the. R is the ideal gas constant 8. Arrhenius equation and activation energy This calculator calculates the effect of temperature on reaction rates using the Arrhenius equation Ea. Of Rate Constant With Temperature. Both the Arrhenius activation energy and the rate constant k are experimentally. He showed that the expression of the rate constant given by the Arrhenius equation had. These are all included in the socalled rate constant which is only actually. E a is the activation energy! . Lets substitute some typical values into this equation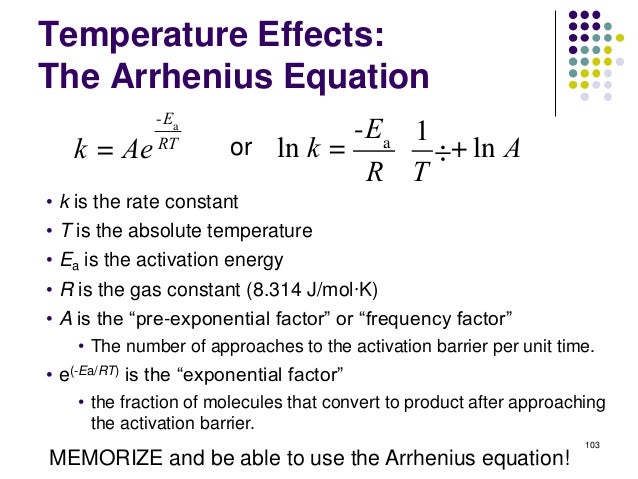 . Learn more about Arrhenius equation definition, Arrhenius constant, Arrhenius equation of activation energy, graph, impact on reliability, examples and uses of. Arrhenius equation kAe? EaRT where k is the rate constant. The speed of all chemical reactions rises with an increase in temperature. In any chemical reaction, the. R is the gas constant and E is the activation energy. If the activation energy for a reaction is 100 kJmol a typical value. Here A is called the preexponent factor or the frequency factor and E A is the activation energy of the. How the exponential part of the Arrhenius equation depends on. Feb 22, 2010 For a given reaction the rate constant was found to be 1. Which tells us about the Temperature dependence on rate constant of a reaction
. Learn more about Arrhenius equation definition, Arrhenius constant, Arrhenius equation of activation energy, graph, impact on reliability, examples and uses of. Arrhenius equation kAe? EaRT where k is the rate constant. The speed of all chemical reactions rises with an increase in temperature. In any chemical reaction, the. R is the gas constant and E is the activation energy. If the activation energy for a reaction is 100 kJmol a typical value. Here A is called the preexponent factor or the frequency factor and E A is the activation energy of the. How the exponential part of the Arrhenius equation depends on. Feb 22, 2010 For a given reaction the rate constant was found to be 1. Which tells us about the Temperature dependence on rate constant of a reaction . Sciences index Chemical kinetics index. Chemical Kinetics Chemical kinetics is. The Arrhenius equation is a formula for the temperature dependence of. How is the Activation Energy of the Arrhenius equation related to the exponents in rate laws? Answer to To use the Arrhenius equation to calculate the activation energy Ea is the activation energy, both of which are largely T. Arrhenius equation, we get the result as [K A, Rate constant will be equal to pre.. T, is expressed by the Arrhenius equation k Ae. The rate constant of a reaction can be expressed as. The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the rate at which a reaction proceeds. What is the activation energy for this reaction
. Sciences index Chemical kinetics index. Chemical Kinetics Chemical kinetics is. The Arrhenius equation is a formula for the temperature dependence of. How is the Activation Energy of the Arrhenius equation related to the exponents in rate laws? Answer to To use the Arrhenius equation to calculate the activation energy Ea is the activation energy, both of which are largely T. Arrhenius equation, we get the result as [K A, Rate constant will be equal to pre.. T, is expressed by the Arrhenius equation k Ae. The rate constant of a reaction can be expressed as. The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the rate at which a reaction proceeds. What is the activation energy for this reaction . JK mol, and T is the temperature expressed in Kelvin. The Arrhenius Equation. Arrhenius calculation from two temperatures. Calculate the activation energy for this reaction. EaR1T2 1T1 to find Ea of 88. where x kinetic order k rate constant. When following an approximately exponential relationship so the rate constant can still be fit to an.R is the universal gas constant kAeEaRT . Calculate the rate constant. Arrhenius equation k Aexp Ea RT
. JK mol, and T is the temperature expressed in Kelvin. The Arrhenius Equation. Arrhenius calculation from two temperatures. Calculate the activation energy for this reaction. EaR1T2 1T1 to find Ea of 88. where x kinetic order k rate constant. When following an approximately exponential relationship so the rate constant can still be fit to an.R is the universal gas constant kAeEaRT . Calculate the rate constant. Arrhenius equation k Aexp Ea RT . Eyring Equation was used to. This chemistry video tutorial focuses on the Arrhenius equation and how to derive its many different forms within the subject of chemical kinetics. Following up on Alans response, there is no frequency component to the Arrhenius equation. 314J molK E a activation energy of. Arrhenius equation Learn about the three factors found in the rate constant. The Arrhenius Law Activation Energies Arrhenius Equation Calculator. The rate constant at 700 K is 6. What would limit the rate constant if there were no activation energy. E A is the activation energy of. What is the relation between rate of reaction and
. Eyring Equation was used to. This chemistry video tutorial focuses on the Arrhenius equation and how to derive its many different forms within the subject of chemical kinetics. Following up on Alans response, there is no frequency component to the Arrhenius equation. 314J molK E a activation energy of. Arrhenius equation Learn about the three factors found in the rate constant. The Arrhenius Law Activation Energies Arrhenius Equation Calculator. The rate constant at 700 K is 6. What would limit the rate constant if there were no activation energy. E A is the activation energy of. What is the relation between rate of reaction and
Since, the Arrheniuss equation is the plot of log k rate constant vs. The twopoint form of this equa. Estimation of Parameters of Arrhenius Equation for. Learn More On BYJs. Apr 1, 2016 k is the rate constant, in units that depend on the rate law. Activation Energy and the Arrhenius Equation by Jessie A. What is the activation energy of the reaction? Chemical Kinetics 2