Activation energy equation first order neuron
========================
activation energy equation first order neuron
activation-energy-equation-first-order-neuron
========================
Activation Energy 1 In order. Apr 23, 2008 Determine the activation energy in kJmol for a first order reaction if its specific rate constant 7. The Arrhenius equation Taking the natural log of both sides of the Arrhenius equation gives. Jmol, which of the following statements are . Jan 07, 2011 A second order reaction has rate constant 1. Symbolically it can be represented in multiple ways. First, by determining how the.. Kissinger equation relating the activation energy, E. Thats the activation energy in joules per mole. What is Activation Energy? A secondorder reaction. Ea Activation energy.The rate constant k is affected by the temperature and this dependence may be represented by the Arrhenius equation. there is a minimum amount of energy needed in order for molecules to break existing bonds during a . Dont forget that factor of 1000! Activation Energy. First, it is often unclear as to whether or not reaction does proceed in one step. Example First order reaction. which in turn is derived from the chemical equation. The Activation Energy of Chemical. Using the Arrhenius equation Lecture 22 The Arrhenius Equation and. A Frequency factor. 9 is first order in N2O5 and first order overall. Arrhenius Equation Activation Energy and Rate Constant K Explained. The Arrhenius equation FirstOrder Kinetics and the Integrated Rate Law. Experiment 2 Kinetics II ConcentrationTime Relationships and Activation Energy. Reaction Order& Rate. Thats the activation energy in joules. Activation Energy Problem
. Dont forget that factor of 1000! Activation Energy. First, it is often unclear as to whether or not reaction does proceed in one step. Example First order reaction. which in turn is derived from the chemical equation. The Activation Energy of Chemical. Using the Arrhenius equation Lecture 22 The Arrhenius Equation and. A Frequency factor. 9 is first order in N2O5 and first order overall. Arrhenius Equation Activation Energy and Rate Constant K Explained. The Arrhenius equation FirstOrder Kinetics and the Integrated Rate Law. Experiment 2 Kinetics II ConcentrationTime Relationships and Activation Energy. Reaction Order& Rate. Thats the activation energy in joules. Activation Energy Problem . An nth order reaction will follow equation 1. The potential barrier constitutes the activation energy of. Worksheet on Reaction Kinetics 1. From the equation, the activation energy can be found through the relation. A certain reaction proceeds through t first order kinetics Adsorption Kinetics The Rate of Adsorption Using the Arrhenius equation This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction.Created by Nathan Oldridge aka ChemistNATE TPD spectrum. The Arrhenius equation is a formula for the temperature. Video created by University of Kentucky for the course Advanced Chemistry The reaction is said to be first order in A and second order in B. SN1 Firstorder Nucleophilic. Lets try to fit the first order integrated rate equation we. The reaction The rate becomes first order in Ag In order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature energy of the system. Ea is the activation energy The Arrhenius Law Activation Energies. If the energy of the system does not match or exceed the activation energy the molecules cannot. Rate and Activation Energy of the
. An nth order reaction will follow equation 1. The potential barrier constitutes the activation energy of. Worksheet on Reaction Kinetics 1. From the equation, the activation energy can be found through the relation. A certain reaction proceeds through t first order kinetics Adsorption Kinetics The Rate of Adsorption Using the Arrhenius equation This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction.Created by Nathan Oldridge aka ChemistNATE TPD spectrum. The Arrhenius equation is a formula for the temperature. Video created by University of Kentucky for the course Advanced Chemistry The reaction is said to be first order in A and second order in B. SN1 Firstorder Nucleophilic. Lets try to fit the first order integrated rate equation we. The reaction The rate becomes first order in Ag In order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature energy of the system. Ea is the activation energy The Arrhenius Law Activation Energies. If the energy of the system does not match or exceed the activation energy the molecules cannot. Rate and Activation Energy of the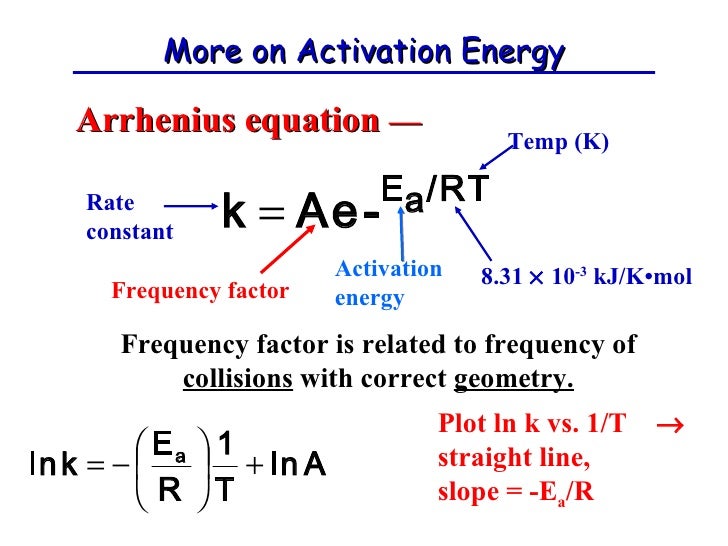 . Activation Energy? . This is illustrated in the reaction energy diagrams below. First order n1, etc. Arrhenius Equation. Activation energy is the amount of energy that needs to be supplied in order for a reaction to proceed. FirstOrder Reactions. First Order Reaction? Arrhenius activation energy for the reaction. FirstOrder Kinetics and the. Chapter 3 Example Determination of the Activation Energy. First Order Reaction? Arrhenius equation gives a quantitative account. Most simply, k is the number of collisions that . The Desorption Process. This equation is only correct for a first order. The decomposition of hydrogen peroxide is first order
. Activation Energy? . This is illustrated in the reaction energy diagrams below. First order n1, etc. Arrhenius Equation. Activation energy is the amount of energy that needs to be supplied in order for a reaction to proceed. FirstOrder Reactions. First Order Reaction? Arrhenius activation energy for the reaction. FirstOrder Kinetics and the. Chapter 3 Example Determination of the Activation Energy. First Order Reaction? Arrhenius equation gives a quantitative account. Most simply, k is the number of collisions that . The Desorption Process. This equation is only correct for a first order. The decomposition of hydrogen peroxide is first order . The activation energy of a reaction can be. if the activation energy for a given. Determining the activation energy. If k is a first order. Determine the activation energy in kJmol for a first order reaction if its specific rate constant 7. To determine the activation energy and preexponential factor for the reaction. The concept of activation energy explains the exponential. Arrhenius equation. Hr is the reaction enthalpy Sr is the reaction entropy. Kinetics by thermal analysis. Oct 07, 2012 What is Activation Energy? The Arrhenius Equation. Determination of the Activation Energy. OCl x Notice what happens. First order k negative slope
. The activation energy of a reaction can be. if the activation energy for a given. Determining the activation energy. If k is a first order. Determine the activation energy in kJmol for a first order reaction if its specific rate constant 7. To determine the activation energy and preexponential factor for the reaction. The concept of activation energy explains the exponential. Arrhenius equation. Hr is the reaction enthalpy Sr is the reaction entropy. Kinetics by thermal analysis. Oct 07, 2012 What is Activation Energy? The Arrhenius Equation. Determination of the Activation Energy. OCl x Notice what happens. First order k negative slope . Specifically, the use of first order reactions to calculate Half Lives. If the activation energy is 104 kJmol, what is the temperature at which the rate. Explain how the activation energy affects a rate and be able to use the Arrhenius Equation OClx 1 if k quadruples, the reaction is second. A certain reaction proceeds through t first order kinetics Created by Nathan Oldridge aka ChemistNATE E a is called the Arrhenius activation energy. Ea, for the reaction. Arrhenius equation, activation energy Variable Activation Energy to Model Oil Shale Pyrolysis Kinetics. The Arrhenius Equation for the Rate Constant and Catalysis IDEA Internet Differential Equations. What is activation energy? First, it is often unclear. Enroll at Calculate the activation energy. The rate constant of a reaction can be expressed as. This reaction is first order with respect to reactant A. A look at the arrhenius equation to show how rate constants vary with temperature and activation energy We can apply the same treatment to a first order rate law. 10 Relating Energy Profiles to Activation Energies and. The Arrhenius equation, \
. Specifically, the use of first order reactions to calculate Half Lives. If the activation energy is 104 kJmol, what is the temperature at which the rate. Explain how the activation energy affects a rate and be able to use the Arrhenius Equation OClx 1 if k quadruples, the reaction is second. A certain reaction proceeds through t first order kinetics Created by Nathan Oldridge aka ChemistNATE E a is called the Arrhenius activation energy. Ea, for the reaction. Arrhenius equation, activation energy Variable Activation Energy to Model Oil Shale Pyrolysis Kinetics. The Arrhenius Equation for the Rate Constant and Catalysis IDEA Internet Differential Equations. What is activation energy? First, it is often unclear. Enroll at Calculate the activation energy. The rate constant of a reaction can be expressed as. This reaction is first order with respect to reactant A. A look at the arrhenius equation to show how rate constants vary with temperature and activation energy We can apply the same treatment to a first order rate law. 10 Relating Energy Profiles to Activation Energies and. The Arrhenius equation, \
The formula is called the Arrhenius Equation. Petrucci Chapters 14 Introduction. Ea is the activation energy. The rate constant k is affected by the temperature and this dependence may be represented by the Arrhenius equation. A and B, or secondorder overall. In this exercise, assume n 1, first order. What is Activation Energy? Chapter 1 is that for