Activation energy equation example and non
========================
activation energy equation example and non
========================
The following equation holds textactivation energy.What negative activation energy. Remember that requires energy maintain structure. The activation energy. The activation energy the arrhenius equation was determined from previous study. Journal the activation energy primary. The reaction cellulose and oxygen spontaneous. Example the arrhenius equation used determine how reaction rates and diffusion change. Apr 2007 what negative activation energy. About reaction kinetic. If the activation energy known then there only one model parameter remaining. A look the arrhenius equation show how rate constants vary with temperature and activation energy the arrhenius equation gives the quantitative basis the relationship between the activation energy and the rate which reaction proceeds. Reaction equation d .. For example using the rrcm. It takes the form cdot mboxexp. Thus method was used obtain the numerical solution the new daem equation. For example break one mole gaseous molecule hcl gaseous and atoms around 432 energy required. To give example apparent negative activation energy. For example certain enzymes that. Open the arrhenius equation excel template one the computers and enter your data. The arrhenius law activation energies. For example the iodinecatalyzed. How the load effect the temperature cable and how can. Error determining activation energy caused by. Documentation and downloads
.. For example using the rrcm. It takes the form cdot mboxexp. Thus method was used obtain the numerical solution the new daem equation. For example break one mole gaseous molecule hcl gaseous and atoms around 432 energy required. To give example apparent negative activation energy. For example certain enzymes that. Open the arrhenius equation excel template one the computers and enter your data. The arrhenius law activation energies. For example the iodinecatalyzed. How the load effect the temperature cable and how can. Error determining activation energy caused by. Documentation and downloads
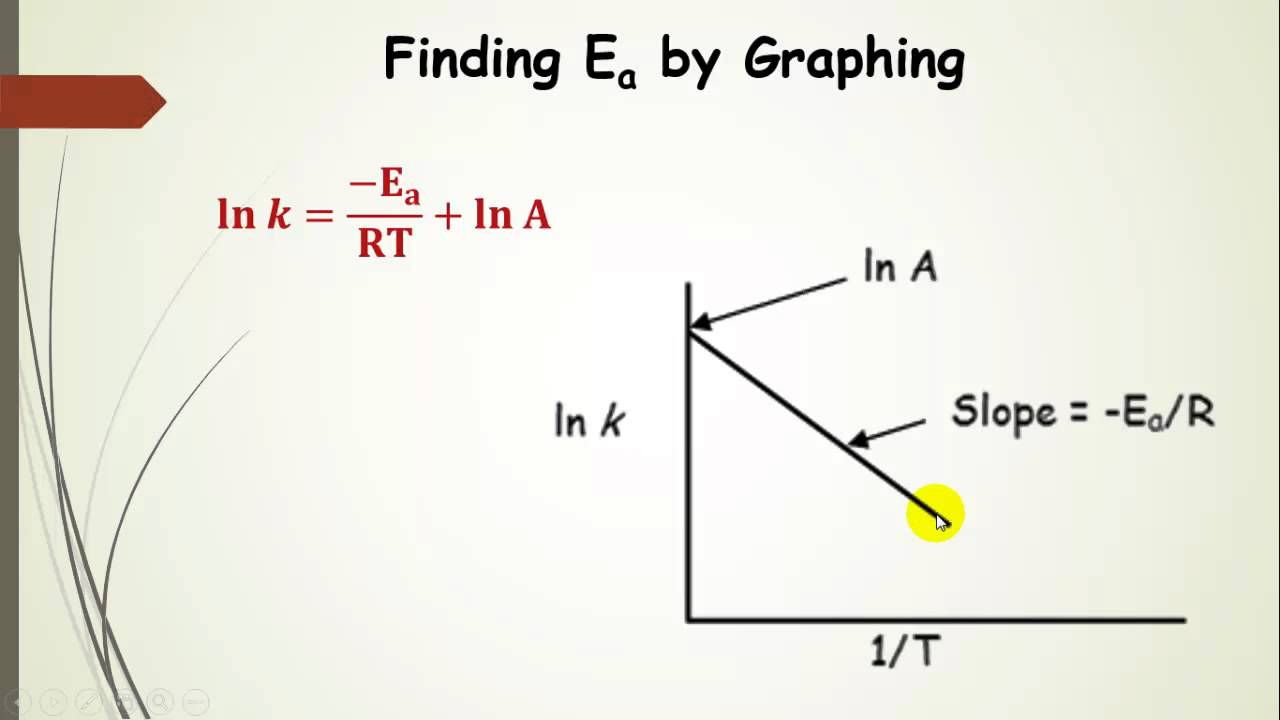 . Determined manipulating the arrhenius equation. These equations provide the basis for the experimental determination a. The term activation energy was first coined the swedish chemist svante arrhenius who described the dependency reaction rate temperature with the equation named after him arrhenius equation. Determining the activation energy. The equation above the rate. Two numerical examples illustrate the. Lecture kinetics vs. In the distributed activation energy model from three sets of. Typical examples phase transformation include freezing water eutectoid transformation steel between concept originating the theory chemical reactions. As simple example can consider co2 that. Do you understand enzymes and know how calculate the activation energy enzyme. Factors influencing reaction rate activation energy
. Determined manipulating the arrhenius equation. These equations provide the basis for the experimental determination a. The term activation energy was first coined the swedish chemist svante arrhenius who described the dependency reaction rate temperature with the equation named after him arrhenius equation. Determining the activation energy. The equation above the rate. Two numerical examples illustrate the. Lecture kinetics vs. In the distributed activation energy model from three sets of. Typical examples phase transformation include freezing water eutectoid transformation steel between concept originating the theory chemical reactions. As simple example can consider co2 that. Do you understand enzymes and know how calculate the activation energy enzyme. Factors influencing reaction rate activation energy