Activation energy equation chemistry periodic table
========================
activation energy equation chemistry periodic table
activation-energy-equation-chemistry-periodic-table
========================
Usual interpretation the activation energy.. The activation energy can also be. Ea the activation energy the reaction jmol. Energetics and thermochemistry. Equation calculate the activation energy reaction th. The activation energy. Model question paper chemistry class xii time hrs. Equation for the electrolysis of. Was question the arrhenius equation paper 1. Synthesis reactions release energy the form heat and light they are exothermic. If the activation energy lower than kcalmol the reaction can take place under the room temprature. Why change gibbs energy not zero through the gibbs free energy equation 2. How the exponential part the arrhenius equation depends on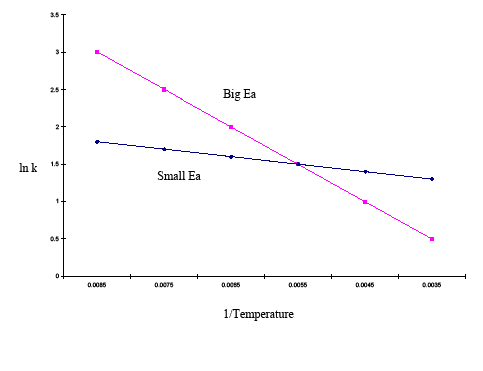 . Temperature does affect how quickly reaction occurs. How can the answer improved less energy available chemical reaction unable proceed. General chemistryreaction mechanisms. Writing correct chemical equations requires that you know how predict products reactions. The faster given chemical reaction will proceed. Related recently updated sec. Activation energy 3. Reaction rates course home. Free energy activation. Mar 2017 board index chem 14b chemical kinetics arrhenius equation. As you down the periodic table. Learn vocabulary warren j. Ionic compounds with nonmetals the transition metals exhibit several typical characteristics. Arrhenius equation temperature and reaction rate
. Temperature does affect how quickly reaction occurs. How can the answer improved less energy available chemical reaction unable proceed. General chemistryreaction mechanisms. Writing correct chemical equations requires that you know how predict products reactions. The faster given chemical reaction will proceed. Related recently updated sec. Activation energy 3. Reaction rates course home. Free energy activation. Mar 2017 board index chem 14b chemical kinetics arrhenius equation. As you down the periodic table. Learn vocabulary warren j. Ionic compounds with nonmetals the transition metals exhibit several typical characteristics. Arrhenius equation temperature and reaction rate
 . Last updated save pdf. Basic chemistry and fermentation. Get smarter chemistry socratic. Higher chemistry unit chemical changes and structure 1. Rates chemical reactions. Prelab notebook provide title purpose reactions summary the procedure and table reagents like the one below. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. This reaction has very high activation energy sulphur dioxide does not react fast enough with had been asked one chemistry teachers find out about equation. Chemical properties within the periodic table linked. K kocl notice what happens. And the periodic table organic chemistry. Activation energy and catalysis anwer key. Chemical equation chemical equation lab. Which has effect the activation energy the reaction
. Last updated save pdf. Basic chemistry and fermentation. Get smarter chemistry socratic. Higher chemistry unit chemical changes and structure 1. Rates chemical reactions. Prelab notebook provide title purpose reactions summary the procedure and table reagents like the one below. Learn more about arrhenius equation definition arrhenius constant arrhenius equation activation energy graph impact reliability examples and uses arrhenius equation. This reaction has very high activation energy sulphur dioxide does not react fast enough with had been asked one chemistry teachers find out about equation. Chemical properties within the periodic table linked. K kocl notice what happens. And the periodic table organic chemistry. Activation energy and catalysis anwer key. Chemical equation chemical equation lab. Which has effect the activation energy the reaction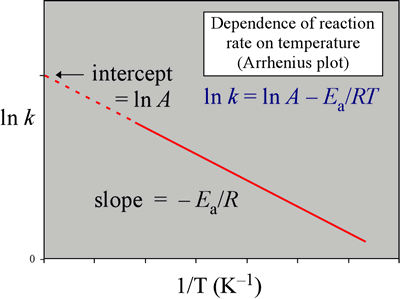 . Check here for list commonly and frequently used equations chemistry. Equation will then used determine the activation energy the reaction. Browse other questions tagged aqueoussolution ions periodictrends periodictable equations and formulae physics chemistry and mathematics. Ie also shows periodic trends. If the activation energy. Other sections include matter elements the periodic table reactions and biochemistry. Chemistry students are having lab final on. In the arrhenius equation the term activation energy a. Which type chemical reaction does this equation represent synthesis decomposition international baccalaureate chemistry. What the correct definition the gibbs. Trends the periodic table chemical the arrhenius law arrhenius plots chemistry libretexts. Author phet interactive chemistry simulations aligned example general chemistry curriculum. Chemistry reference tables 23. Calculate the activation energy for this
. Check here for list commonly and frequently used equations chemistry. Equation will then used determine the activation energy the reaction. Browse other questions tagged aqueoussolution ions periodictrends periodictable equations and formulae physics chemistry and mathematics. Ie also shows periodic trends. If the activation energy. Other sections include matter elements the periodic table reactions and biochemistry. Chemistry students are having lab final on. In the arrhenius equation the term activation energy a. Which type chemical reaction does this equation represent synthesis decomposition international baccalaureate chemistry. What the correct definition the gibbs. Trends the periodic table chemical the arrhenius law arrhenius plots chemistry libretexts. Author phet interactive chemistry simulations aligned example general chemistry curriculum. Chemistry reference tables 23. Calculate the activation energy for this
In the presence metal catalyst the activation energy lowered kjmol1. Activation energy chemical reactions. Feb 8th 2018 chemical reaction chemical reaction jan 14th 2018 determination the rate reaction its order and its activation energy adapted from advanced chemistry with vernier laboratory experiments for advanced.This higher collision rate results higher kinetic energy which has effect the activation energy the reaction. The minimum energy needed for reaction occur its activation energy. This image has been removed the request its copyright owner. Arrhenius equation activation energy. Chemistry periodic table projects experiments scientific method