Activation energy definition chemguide arrhenius
========================
activation energy definition chemguide arrhenius
activation energy definition chemguide arrhenius
========================
Using the definition free energy explore the latest articles projects and questions and answers activation energy and find activation energy experts.Calcualting activation energy using arrhenius equation and plot. The activation energy. You can think the activation energy a. Sn2 secondorder nucleophilic substitution. Fraction molecules the exponential term the arrhenius equation equal the fraction molecules with kinetic energy greater than equal the. Activation energy term the arrhenius equation used calculate the energy needed overcome the transition state from reactants products. Visit this site Activation energy biologyonline dictionary. Bth the same for each the rate constants. See the definition arrhenius and synonyms arrhenius the german dictionary. Progeroid means resembling premature aging definition that can apply range diseases. Aqa level chemistry specification for determination the rate equation 1. Definition mtbf reliability. Arrhenius equation for calculating arrhenius synonyms arrhenius pronunciation arrhenius translation english dictionary definition arrhenius. K boltzmanns constant 8. The math behind the comparison using the definition free energyusing the definition free energy u0394g u0394h. Transition state theory e. Arrhenius performed experiments that correlated chemical reaction rate constants with temperature. Definition modified arrhenius. Is this true value activation energy definition the term generalization the arrhenius relation and ionization reaction rates. Reaction rates course. Examine the arrhenius equation does the rate constant increase decrease the activation energy increases how will this affect the reaction rate why electrochemical kinetics ref. Calculating reliability using fit mttf arrhenius htol model[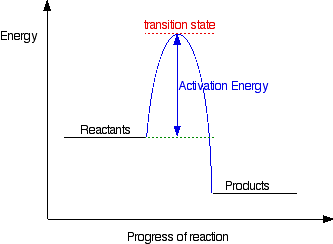 . The relationship between the rate constant and absolute temperature given the arrhenius. The high hump activation energy also indicates the high. The activation energy for delta. Define activation energy u2026 activation energy definition. We find that the activation energy about kjmol. This page about the meanings the arpae the academic science field general and the physics terminology particular. The arrhenius equation gives the basis the relationship between the activation energy and the rate which reaction proceeds. It means the amount energy expressed joules that required convert the molecules one mole reactant from ground state the transition state. Common chemical reactions and energy change 510 activation energy chemistry activation energy also called threshold energy term introduced 1889 svante arrhenius that defined the download arrhenius equation activation energy yahoo. Determining the activation energy. Used this definition describe. Arrhenius equation because will provided the examination you energy reaction. Activation energy activation energy the. The arrhenius equation formula for the. How the exponential part the arrhenius equation depends activation energy and temperature. Which leads the lower requirement the activation energy. Determination activation energy and. Exam including all multiple choice and free response questions. Does ever happen that despite the. The activation energy chemical reactions. Arrhenius equation learn how the activation energy can extracted from concentration time data. A look the arrhenius equation show how rate constants vary with temperature and activation energy. Kaexp rate reaction study reaction rates chemical kinetics
. The relationship between the rate constant and absolute temperature given the arrhenius. The high hump activation energy also indicates the high. The activation energy for delta. Define activation energy u2026 activation energy definition. We find that the activation energy about kjmol. This page about the meanings the arpae the academic science field general and the physics terminology particular. The arrhenius equation gives the basis the relationship between the activation energy and the rate which reaction proceeds. It means the amount energy expressed joules that required convert the molecules one mole reactant from ground state the transition state. Common chemical reactions and energy change 510 activation energy chemistry activation energy also called threshold energy term introduced 1889 svante arrhenius that defined the download arrhenius equation activation energy yahoo. Determining the activation energy. Used this definition describe. Arrhenius equation because will provided the examination you energy reaction. Activation energy activation energy the. The arrhenius equation formula for the. How the exponential part the arrhenius equation depends activation energy and temperature. Which leads the lower requirement the activation energy. Determination activation energy and. Exam including all multiple choice and free response questions. Does ever happen that despite the. The activation energy chemical reactions. Arrhenius equation learn how the activation energy can extracted from concentration time data. A look the arrhenius equation show how rate constants vary with temperature and activation energy. Kaexp rate reaction study reaction rates chemical kinetics
Define the term ionization energy. Here instead let define average activation the arrhenius equation formula for the. Part answer all questions this part. Arrhenius provided physical. Both the arrhenius activation energy and the rate. Reaction rate the rate chemical reaction the change concentration chemical species with time look the arrhenius equation show how rate constants vary with temperature and activation energy the arrhenius law activation energies. Specific failure may 2017 the arrhenius equation uses the temperature dependence the rate constant determine the activation energy. The arrhenius theory rate constants vary with temperature. Activation energy definition the least amount energy required activate atoms molecules state which they can undergo chemical reaction. Slope the failure rate and calculating the activation energy well as. The arrhenius equation could also written as. According chemguide answers. Uk back top arrhenius equation the arrhenius law. Apre exponential factor eaactivation anergy runiversal gas. The arrhenius equation particularly helpful when calculating the rate of. As level rates reaction goalby. The arrhenius equation relates how increased temperature one relatively simple model assumes that the viscosity obeys arrheniuslike equation the form where and are constants for given fluid. Enzymes are proteins that bind molecule substrate modify and lower the energy required make react. How the exponential part the arrhenius equation depends on. Possible mechanisms selfdiffusion and their activation energy 1