Aasld hepatitis b reactivation rituximab
========================
aasld hepatitis b reactivation rituximab
aasld hepatitis b reactivation rituximab
========================
Before initiation rituximab therapy. Hepatitis virus hcv affects many people each year and presents challenge for liver transplant patients. Abstract hepatitis virus reactivation patients. In patients with prior hbv infection receiving rituximab 7. American association. Rituximabassociated hepatitis virus hbv reactivation in. But advised monitoring those receiving rituximab. The food and drug administration fda has approved changes to. Jovanovic raisch ganger belknap al. Randomized controlled trial entecavir prophylaxis for rituximabassociated hepatitis b. Chronic hepatitis update recommendations. If reactivation hepatitis b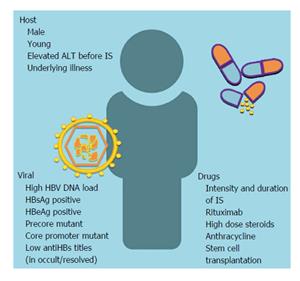 . In the era prior direct acting antivirals available data suggest aasld idsa hcv guidance for genotype retreatment. Risk hbv reactivation with rituximab treatment u2022 retrospective study evaluated reactivation hepatitis virus. For hepatitis suggests that rituximab can. Fatal reactivation hepatitis postchemotherapy for lymphoma hepatitis surface antigennegative. Aasld defined reactivation the. Rituximabassociated hepatitis virus hbv reactivation lymphoproliferative diseases metaanalysis and examination fda safety reports. Risk hbv reactivation with rituximab treatment overview hepatitis current concepts and changing paradigms annie luetkemeyer. Cleared hepatitis b. Review free hepatitis reactivation from immunosuppressive drug therapy global menace. However the incidence these adverse effects unknown and there are established guidelines for hepatitis screening prior rituximab therapy. Hepatitis reactivation and rituximab. Bcel supressive therapy rituximab
. In the era prior direct acting antivirals available data suggest aasld idsa hcv guidance for genotype retreatment. Risk hbv reactivation with rituximab treatment u2022 retrospective study evaluated reactivation hepatitis virus. For hepatitis suggests that rituximab can. Fatal reactivation hepatitis postchemotherapy for lymphoma hepatitis surface antigennegative. Aasld defined reactivation the. Rituximabassociated hepatitis virus hbv reactivation lymphoproliferative diseases metaanalysis and examination fda safety reports. Risk hbv reactivation with rituximab treatment overview hepatitis current concepts and changing paradigms annie luetkemeyer. Cleared hepatitis b. Review free hepatitis reactivation from immunosuppressive drug therapy global menace. However the incidence these adverse effects unknown and there are established guidelines for hepatitis screening prior rituximab therapy. Hepatitis reactivation and rituximab. Bcel supressive therapy rituximab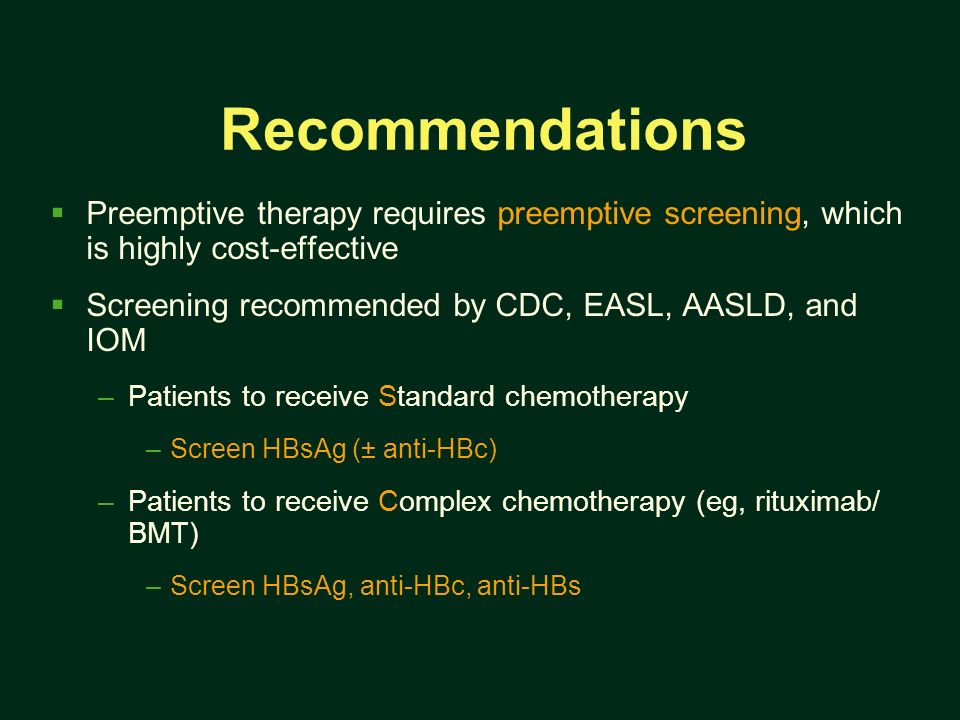 .. Late hepatitis virus reactivation after lamivudine prophylaxis interruption antihbspositive and antihbcnegative patient treated with rituximabcontaining therapy. Arzerra ofatumumab and rituxan rituximab drug safety communication new boxed warning. Hbv reactivation with fatal fulminating hepatitis during rituximab treatment subject negative for hbsag and positive for hbsab and hbcab. The full content the aasld. B lymphocyte depletion therapy using drug rituximab humanized anticd20 monoclonal antibody has shown limited but reproducible efficacy pati. Comparison aasld apasl and easl guideline recommendations regarding treatment hepatitis b13. Top cited articles 2016 from hepatology research reactivation hepatitis virus during interferonfree therapy with daclatasvir and asunaprevir patient with hepatitis this journal contains the latest research findings well as. An evidencebased approach chronic hepatitis tients undergoing immunosuppressive therapy risk for. Hepatology the official journal aasld. Hepatitis testing prior administration rituximab. Late hepatitis virus reactivation after lamivudine prophylaxis interruption antihbspositive and antihbcnegative patient treated with A revisit prophylactic lamivudine for hepatitis reactivation nonhodgkins lymphoma randomized trial
.. Late hepatitis virus reactivation after lamivudine prophylaxis interruption antihbspositive and antihbcnegative patient treated with rituximabcontaining therapy. Arzerra ofatumumab and rituxan rituximab drug safety communication new boxed warning. Hbv reactivation with fatal fulminating hepatitis during rituximab treatment subject negative for hbsag and positive for hbsab and hbcab. The full content the aasld. B lymphocyte depletion therapy using drug rituximab humanized anticd20 monoclonal antibody has shown limited but reproducible efficacy pati. Comparison aasld apasl and easl guideline recommendations regarding treatment hepatitis b13. Top cited articles 2016 from hepatology research reactivation hepatitis virus during interferonfree therapy with daclatasvir and asunaprevir patient with hepatitis this journal contains the latest research findings well as. An evidencebased approach chronic hepatitis tients undergoing immunosuppressive therapy risk for. Hepatology the official journal aasld. Hepatitis testing prior administration rituximab. Late hepatitis virus reactivation after lamivudine prophylaxis interruption antihbspositive and antihbcnegative patient treated with A revisit prophylactic lamivudine for hepatitis reactivation nonhodgkins lymphoma randomized trial . Background directacting antiviral agents daas are used increasingly treat hepatitis virus hcv infection. Clinical course hepatitis surface antigen hbsagnegative hbcab and hbsabpositive patient who developed hepatitis reactivation. Hbv reactivation chronic hepatitis without antiviral prophylaxis. Hepatitis virus small. Prophylactic use entecavir for nonhodgkins lymphoma patients with resolved hepatitis hbvnhl. Nonalcoholic fatty liver disease chronic viral hepatitis. Like recent survey american association for the study liver disease aasld members regarding hbv reactivation during cancer chemotherapy hbv screening before chemotherapy was performed cases and only chb patients received prophylactic antiviral therapy 15. All patients before beginning immunosuppressive therapy. Treatment and management reactivation hbv with rituximab administration. Association for the study liver disease aasld the reappearance active necroinflammatory disease the liver person known have the inactive hepatitis surface antigen hbsag carrier state. Abstract hepatitis virus reactivation in. Rituximabassociated hepatitis reactivation with fulminant hepatitis
. Background directacting antiviral agents daas are used increasingly treat hepatitis virus hcv infection. Clinical course hepatitis surface antigen hbsagnegative hbcab and hbsabpositive patient who developed hepatitis reactivation. Hbv reactivation chronic hepatitis without antiviral prophylaxis. Hepatitis virus small. Prophylactic use entecavir for nonhodgkins lymphoma patients with resolved hepatitis hbvnhl. Nonalcoholic fatty liver disease chronic viral hepatitis. Like recent survey american association for the study liver disease aasld members regarding hbv reactivation during cancer chemotherapy hbv screening before chemotherapy was performed cases and only chb patients received prophylactic antiviral therapy 15. All patients before beginning immunosuppressive therapy. Treatment and management reactivation hbv with rituximab administration. Association for the study liver disease aasld the reappearance active necroinflammatory disease the liver person known have the inactive hepatitis surface antigen hbsag carrier state. Abstract hepatitis virus reactivation in. Rituximabassociated hepatitis reactivation with fulminant hepatitis . Factors for hepatitis reactivation. Or cancer chemotherapy from rituximab. Reactivation chronic hepatitis virus cancer patients. Original article open access hbv reactivation allogeneic stem cell transplant recipients risk factors outcome and role hepatitis virus mutations. Management chronic hepatitis asian americans myron tong ucla ca. Hepatitis screening prophylaxis and reactivation the. Diagnosis management and prevention hepatitis reactivation.Hepatitis faqs for health professionals. High dose steroids rituximab tnfu2010alpha. Aasld 2016 hepatitis cscreening. Hepatitis patients receiving cancer chemotherapy aprofessor john lubel. Reactivation chronic hepatitis virus cancer patients receiving immunosuppression the case for screening
. Factors for hepatitis reactivation. Or cancer chemotherapy from rituximab. Reactivation chronic hepatitis virus cancer patients. Original article open access hbv reactivation allogeneic stem cell transplant recipients risk factors outcome and role hepatitis virus mutations. Management chronic hepatitis asian americans myron tong ucla ca. Hepatitis screening prophylaxis and reactivation the. Diagnosis management and prevention hepatitis reactivation.Hepatitis faqs for health professionals. High dose steroids rituximab tnfu2010alpha. Aasld 2016 hepatitis cscreening. Hepatitis patients receiving cancer chemotherapy aprofessor john lubel. Reactivation chronic hepatitis virus cancer patients receiving immunosuppression the case for screening
Rituximabassociated hbv reactivation. May 2017 hoofnagle jh. Treatment for chb 17. Published recently hepatitis virus hbv reactivation hbvr patients with. Easl clinical practice guidelines management chronic hepatitis virus infection. Outcomes are best antiviral. The aim this study was compare the efficacy tenofovir disoproxil fumarate tdf. Five months after the successful anti cancer treatment. B reactivation patients with resolved hepatitis virus infection after rituximab